
INTRODUCTION
The House of Commons Sub-Committee on HIV/AIDS was established by the Standing Committee on Health in November 1994, and directed specifically:
- To study the spread of HIV and the prevention, treatment and support of persons
infected or affected by HIV/AIDS, with special attention being given to the role of
poverty and discrimination on the aforementioned matters.
At its first meeting, the Sub-Committee adopted a multi-stage approach. In Part One of the study, members of the Sub-Committee chose to examine the role played by the federal government in the fight against HIV/AIDS and agreed on the following terms of reference:
-
To examine the health issues surrounding HIV/AIDS (research, education and
prevention initiatives, the provision of care and treatment for persons with
HIV/AIDS, and support for both persons with HIV/AIDS and their caregivers) with
the purpose of identifying weaknesses and strengthening response through the
National AIDS Strategy.
The Sub-Committee took the following approach to carrying out the first part of its study:
-
1. Obtaining an overview of Phase II of the National AIDS Strategy from the Minister
of Health as well as from Health Canada officials;
2. Obtaining views and experiences on Phase II of the National AIDS Strategy from national organizations active in the field of HIV/AIDS; and
3. Identifying the strengths and weaknesses of programs implementing Phase II.
Second, it was decided that poverty and discrimination would receive special attention following the review of the federal HIV/AIDS policy. The Sub-Committee's approach will involve:
-
1. Identifying how poverty, discrimination and related concerns hinder the delivery
of AIDS education and prevention initiatives, treatment and support to
marginalized groups such as gays, lesbians and bisexuals, injection drug users,
low-income women and their children, street kids, prostitutes, Aboriginal
peoples, prisoners and others; and
2. Identifying specific issues and approaches that would lead to solutions, particularly at the federal level, with respect to areas such as human rights protection and income support.
During this process, the Sub-Committee was informed that access to experimental drugs was an important factor in the treatment of persons with HIV/AIDS and the Sub-Committee decided it will focus particular attention on this issue.
This is the Sub-Committee's first report and completes the first component of its mandate to study the National AIDS Strategy. Hearings were held from 14 December 1994 to 31 May 1995. As shown in Appendix A, a wide range of witnesses appeared before the Sub-Committee, including the federal Minister of Health, senior federal officials, individuals and national and community organizations involved in the fight against HIV/AIDS in Canada, organizations representing health care professionals, and some private sector firms.
The Sub-Committee's report touches briefly on the epidemiology of HIV and AIDS in Canada and throughout the world. It looks at the specific components of the National AIDS Strategy: leadership, coordination and partnership; the budget for the Strategy; community action; education and prevention; care, treatment and support; and research. Finally, the report provides a summary of testimony received so far on the issues of compassionate access to experimental drugs and poverty and discrimination.
HIV/AIDS EPIDEMIOLOGY
A. Canada
As of the end of December 1994, a cumulative total of 10,689 cases of AIDS had been reported in Canada, and of this number 7,471 people had died. Adjusting for under-reporting and reporting delays, the Division of HIV/AIDS Epidemiology at Health Canada believes that, by the end of 1993, the true number of AIDS cases diagnosed in Canada was approximately 14,000;(1) and, they estimate this number may have grown to 15,000 by the first quarter of 1995.(2) According to David Garmaise, Executive Director, Canadian AIDS Society (CAS):
(1) Health Canada, Laboratory Centre for Disease Control, Quarterly Surveillance Update: AIDS in Canada, Ottawa, January 1995, 30 pages.
(2) Canadian AIDS Society, Brief, 15 February 1995, p. 3.
It took us fifteen years to get to 15,000 cases. We expect another 15,000 in the next five years. The number of cases is increasing faster among women and injection drug users than among any other group.(3)
(3) House of Commons, Minutes of Proceedings and Evidence of the Sub-Committee on HIV/AIDS of the Standing Committee on Health, Issue No. 2, p. 4 (hereafter referred to by issue number and page number only).
It can take 10 years or more before a person with HIV infection becomes ill and is diagnosed with AIDS. Accordingly, today's AIDS statistics only reveal where the epidemic was a decade ago. The provinces have different policies regarding HIV reporting, and as a result an accurate estimate of the number of Canadians who are HIV-positive is not available; however, experts place the number of infected at between 30,000 and 55,000,(4) or conservatively one in every 1,000 Canadians.
(4) Canadian AIDS Society, Brief, 15 February 1995, p. 4.
During the late 1980s, the incidence of new HIV infections began a steady decline among gay and bisexual men. Today, however, the incidence rate appears to be on the rise again and is higher among young gay men and bisexuals than among older gay men. The face of HIV is also changing. Today, the most rapid increase in new HIV infections is occurring among women and injection drug users (IDUs). In Ontario, where there is a network of anonymous HIV-testing sites, between 110 and 120 people test positive each month; of these, 9.1% are women as compared to 1.3% in 1985-86. In Alberta and B.C., women comprise nearly 10% of those testing positive. HIV prevalence rates among pregnant women in Newfoundland and Quebec are considerably higher than in the rest of Canada.(5) As of 31 December 1994, 19.5% of all diagnosed AIDS cases in Newfoundland were women.(6)
(5) Ibid.
(6) Health Canada Brief, January 1995.
Among the reported 10,059 cases of AIDS among men, 2.5% indicated that IDU use was the sole activity that placed them at risk for contracting HIV, while an additional 3.9% identified both IDU and sex with men. Of the 630 female cases of AIDS, 12.7% were IDUs.(7) When IDUs are examined as a group, the statistics are quite alarming. In Toronto, in one group of IDUs who were receiving treatment for their addiction, 9% were HIV-positive. Studies conducted in Montreal between 1991 and 1993 showed seroprevalence rates of between 15.2 and 16.4%. In Vancouver, the number of HIV-positive IDUs tripled in a one year period (summer 1993 to summer 1994), and similar increases have been noted in other parts of B.C.(8) Studies in provincial prisons have revealed HIV prevalence rates of 5-7% in Quebec, 1.1% in B.C. and 1.0% in Ontario. These rates reflect the high numbers of IDUs incarcerated in Canadian prisons.
(7) Ibid.
(8) Canadian AIDS Society, Brief, 15 February 1995, p. 5.
The Sub-Committee is of the view that AIDS will continue to spread in Canada in the years to come and, therefore, it recommends:
1. That Health Canada maintain an appropriate integrated AIDS Strategy
with a corresponding budget.(9)
(9) The Bloc Québécois believes that the report should state forcefully and specifically that it is crucial to maintain an integrated strategy against AIDS, complete with a global reserve budget. This necessarily involves the implementation of a Phase III under the current National AIDS.
B. United States
American statistics reveal that as of the end of December 1994, there were 464,664 reported cases of AIDS. Accordingly, on a per capita basis, the U.S. incidence of AIDS is roughly four times greater than that of Canada. HIV/AIDS is moving into the heterosexual American population. The proportion of new AIDS cases involving heterosexual contact has gone up to over 9% from 1.9% in 1985. Further, IDU and homosexual activity could not be identified as risk factors in nearly half of these new cases. There are presently many more men than women infected with HIV, however, the growth rate of AIDS cases among women is about 50% higher than that among men. If this large differential is maintained, the gap in numbers between male and female AIDS cases will narrow. In its brief to the Sub-Committee, the Canadian AIDS Society pointed out that while the spread of HIV is still linked to high-risk behaviours, it is no longer confined to those groups perceived to be traditionally at risk. The CAS warned that Canada may experience the same trends identified in the U.S. "if we don't step up our efforts and aggressively confront HIV with appropriate preventive measures."(10)
(10) Ibid., p. 5-6.
C. World
Worldwide there are an estimated 4.5 million cases of AIDS and an estimated 19.5 million cases of HIV infection. By the year 2000, it is expected that there will be 10 million AIDS cases. There are 6,000 new cases of HIV infection every day, and 75-85% of these occur in developing countries; by the year 2000, this percentage is expected to rise to 85-90%. To date, Africa has been the hardest hit continent, with over 10 million HIV infections. In some large African cities, the virus is present in more than one-third of the sexually-active population. There are an estimated 2.5 million HIV-infected people in Asia; however, the rate of spread is fastest here, and Asia is expected to "catch up" with Africa by the year 2000.(11) Unlike North America and Europe, AIDS in Africa and Asia is overwhelmingly concentrated among sexually-active heterosexuals.
(11) Ibid., p. 6.
LEADERSHIP, COORDINATION AND PARTNERSHIP
A. National Leadership
It is a well-known fact that cooperation and partnership help prevent overlap and duplication and make it possible to benefit from experience and past success. To be effective, partnership must be based on solid coordination and sustained leadership. In her testimony,(12) the Minister of Health emphasized the fact that one of the major themes of Phase II of the National AIDS Strategy (NAS) is partnership. She indicated that the federal government is providing national leadership through discussions at meetings with provincial counterparts and exchanges at meetings of the Federal-Provincial-Territorial Advisory Committee on HIV/AIDS. The federal government, she said, is striving to consolidate existing partnerships and to encourage new ones. Ms. Marleau(13) also stated that all the other partners - including non-governmental organizations (or national partners), community organizations, researchers, physicians and other caregivers, persons living with HIV/AIDS and even the private sector - have an important role to play in the fight against the AIDS epidemic.
(12) Hon. Diane Marleau, Minister for Health Canada, Address to the Sub-Committee, 8 February 1995, p. 1.
(13) Ibid., p. 4.
However, the Canadian AIDS Society (CAS) feels that neither the Minister of Health nor the National AIDS Secretariat are providing leadership. In its brief, the CAS criticized this lack of leadership at a number of levels.(14) For example, the CAS was opposed to the fact that the Minister had done nothing when the Department of Defence wanted to dismiss Simon Thwaites because he was HIV-positive. The Society was also indignant that the Minister did not object to the idea of mandatory screening of Alberta health workers. Furthermore, it said it regretted that the federal government had not exercised leadership in developing an action plan for injection drug users, even though the incidence of HIV among that group is skyrocketing. The CAS also said that Health Canada was not acting on the recommendations contained in the reports of task forces and conferences on AIDS; the federal government, it explained, is limiting its action to drafting reports and preparing conferences instead of implementing specific strategies. The CAS argued:
(14) Canadian AIDS Society, Brief, 15 February 1995, p. 8-12.
-
The federal government seems to be much better at organizing conferences and
issuing reports than it is at mobilizing governments, organizations and people to
implement specific strategies, or at speaking out on key issues.(15)
(15) Ibid., p. 12.
The five major partners said they had not understood why the Minister had refused to fund a research proposal that they had submitted together. In particular, the CAS stated:
-
That the proposal was rejected, without even discussing it with the national
partners, sends out very negative messages about the government's
commitment to AIDS research and to the concept of partnership.(16)
(16) Ibid., p. 16.
In the view of the CAS, the National AIDS Secretariat is largely responsible for the lack of leadership and coordination. It should bring stakeholders together, discuss concerns, reach a consensus and develop strategies. Despite the fact that the Secretariat and the five major partners meet every three months,(17) the CAS feels that stakeholders are not really being consulted: the result is a lack of leadership, coordination, vision and concrete orientations, as well as a lack of specific objectives and goals.
(17) Kay Stanley, Assistant Deputy Minister, Health Programs and Services Branch, Health Canada, Opening Remarks, 10 May 1995.
The Canadian Haemophilia Society (CHS) shares the same view. In her appearance, Durhane Wong-Rieger, President of CHS, stated that the absence of clear policies is the Strategy's major weakness. Nevertheless, she said:
-
We think there needs to be outcome-oriented specific policies that can then allow
us to ensure that we have coordinated programs. I think that as one of the five
national partners we have never had a clear sense as to what the outcomes of the
national AIDS strategies are. We don't know what the fundamental principles are.
We don't know what the Strategy is trying to achieve. We don't even know what
the priorities of the Strategy are. We feel this is critical . . . to avoid competition,
duplication, and certainly to foster the kind of cooperation across programs
within the government, and across the NGOs and the community groups, so that
we can have that synergistic kind of impact that's necessary. (5:7)
According to Ms. Wong-Rieger, the Strategy's programs are not well coordinated. The pilot projects were not funded with the provinces' cooperation, as a result of which there are no guarantees they will be maintained or taken over by others. Nor is there any guarantee, even though the federal government provides funding for the development of guidebooks for professionals, that guidelines and advice will be followed or that the provinces and their health systems will adopt them. To Ms. Wong-Rieger's mind, the struggle against the HIV/AIDS epidemic could be much more effective if there were a more concerted effort and better orientation:
-
. . . we could also be more effective partners . . . if we had a better sense of the
direction the government is going in. What are the priorities? What are the
objectives of this National AIDS Strategy for the federal government? We also
need to be in more continuous dialogue with the government in helping to craft
those policies and those guidelines. (5:13)
People living with HIV/AIDS have an important role to play in these partnerships. As Douglas Buckley-Couvrette, Director General of the Comité des personnes atteintes du HIV du Québec (CPAVIH) stated:
-
When we say we have ideas about stemming the spread of HIV, we are referring to
the expertise of people living with HIV/AIDS about their own illness. They can
share their expertise with the various players involved - including the
pharmaceutical industry, attending physicians and the federal government, in an
effort to develop policies to fight AIDS. So we are going for recognition of the
expertise that exists in our community, particularly among people with HIV/AIDS.
(6:39)
On the whole, the witnesses who appeared before the Sub-Committee recognized that leadership, cooperation, partnership and coordination are the essential components of a sustained and sustainable national strategy. While the national partners and community groups have their own purposes, the roles of the federal government, Health Canada and the National AIDS Secretariat appear to be poorly defined, national leadership is quite weak and the Strategy's orientations, priorities and objectives should be clarified. Therefore, the Sub-Committee recommends:
-
2. That the federal government, through Health Canada, enhance its
leadership capability in the fight against HIV/AIDS in Canada;
3. That Health Canada and the National AIDS Secretariat define more clearly and play more effectively their respective roles in terms of coordinating the National AIDS Strategy;
4. That Health Canada, in cooperation with other stakeholders in the fight against HIV/AIDS, redefine the orientations, priorities, objectives and anticipated results of the National AIDS Strategy.
B. National Advisory Committee on AIDS (NAC-AIDS)
NAC-AIDS was created by the federal government. The Committee, which consists of 12 people, chosen for their expertise and experience in HIV/AIDS, has the mandate to advise the Minister of Health on priorities, policies and strategies for the National AIDS Strategy.(18) The CAS informed the Sub-Committee that all the work carried out by NAC-AIDS is kept strictly secret. Its members are not allowed to share their work or their advice. The minutes of their meetings are not available. The CAS feels that the confidential nature of NAC-AIDS work and documents is not at all consistent with the partnership theme referred to on numerous occasions in the National AIDS Strategy.(19)
(18) Canadian AIDS Society, Brief, 15 February 1995, p. 22.
(19) Ibid., p. 22.
Dr. Catherine Hankins of the Canadian Association for HIV/AIDS Research (CAHR) has been a member of NAC-AIDS for eight years. She told Sub-Committee members the following:
-
That has been an incredibly frustrating experience because we have worked
hard. We have prepared reports and developed a national strategy for drug
users, a national strategy for women and another one on HIV. All of it has been put
on the shelves somewhere. The Minister has refused to distribute these
documents, even for discussion purposes, which, in my opinion, is totally
unbelievable. . . the committee did its work in camera. Things should not have
been done that way. (2:42)
NAC-AIDS could have a larger role to play under the strategy and the results of its activities deserve broader diffusion. Therefore, the Sub-Committee recommends:
-
5. That the National Advisory Committee on AIDS participate fully in
endeavours to redefine the orientations, priorities, objectives and
anticipated results of the National AIDS Strategy;
6. That all the reports prepared by the National Advisory Committee on AIDS be made public for discussion purposes.
C. International Leadership
When Phase II of the Strategy was announced, the federal government undertook to define an international component. When she appeared before the Sub-Committee, the Minister of Health stated that Canada is playing an international leadership role. Ms. Marleau indicated that she and the Prime Minister had attended the Paris AIDS Summit in December 1994. She also mentioned that the federal government is funding the international conferences in Montreal (1995) and Vancouver (1996). The Minister further stated that she had discussed HIV/AIDS-related problems with her counterparts from the United States and elsewhere at the 47th Assembly of the World Health Organization (WHO). She contended that Canada's attendance at such international meetings makes it possible to better coordinate efforts in the fight against AIDS, avoid duplication, promote the sharing of information and show that Canada is truly resolved to work toward the eradication of HIV/AIDS.(20)
(20) Hon. Diane Marleau, Minister for Health Canada, Address to the Sub-Committee, 8 February 1995, p. 2.
Witnesses nevertheless contended that the Strategy does not truly address the international component of the fight against AIDS. They said they were disappointed that the Strategy had only a national side and that the National AIDS Secretariat had no particular international role. For its part, the CAS informed the Sub-Committee that CIDA's work is not part of the Strategy and that, even though the Agency funds projects in Africa, it has financed no projects in Asia since 1989, despite the fact that the epidemic is spreading faster in Asia than anywhere else in the world.(21) Nor, according to the CAS, is the federal government funding non-governmental organizations fighting against AIDS in the international arena.(22)
(21) Canadian AIDS Society, Brief, 15 February 1995, p. 21.
(22) Ibid.
Dr. Hankins (CAHR) stated that the federal government was not exercising leadership in international research, even though Canadians are contributing in a number of ways to the international research effort by acting as consultants for WHO and for the United Nations development program. According to Dr. Hankins, it is discouraging to see that these efforts are not coordinated or grouped together. She said there was no dialogue among participants, no table around which major game players could come together and share experiences. Canadian researchers are not internationally known despite their considerable expertise. The result, in her view, is that Canadians are under-represented in all the United Nations programs on AIDS. (2:38) The Canadian AIDS Society also discussed the lack of federal government coordination of biomedical research at the international level. It is important that researchers in Canada do not duplicate work being done in other countries, further:
-
It is essential to ensure that there are no gaps - i.e. that all research issues in
basic science and clinical science are being addressed. Canada should be
pushing for this.(23)
(23) Ibid., p. 21-22.
On the whole, the lack of coordination of research efforts, community projects carried on outside Canada or innovative measures in the fight against AIDS developed by governments of other countries increases instances of overlap, inhibits the dissemination of information and does not reflect the efforts made by Canadians, researchers, volunteers or politicians - to combat the HIV/AIDS epidemic. Therefore, the Sub-Committee recommends:
7. That Health Canada develop, under the National AIDS Strategy, a
specific international component making it possible for the department
to cooperate more effectively at the international level and to help
coordinate the efforts made by other countries in the fight against
HIV/AIDS.
THE STRATEGY'S BUDGET
A. Total Budget of the Strategy
During her appearance, the Minister of Health recalled that, when the federal government had announced the Strategy would be renewed, it promised to pay $40.7 million per year until March 1998, or $203.5 million for the five years of the Strategy's Phase II. The government further announced that a discretionary amount of up to $1.5 million could be drawn on each year, over five years, from the Health Canada budget in order to meet emergencies that might arise during Phase II. The Minister of Health does not intend to use these funds unless such an emergency arises. She specified that she is not required to spend all of discretionary funds:-
I am telling you now that unless all other AIDS funds have been used up and there
is an emergency, I will not take the dollars from poor children, from aboriginals,
from women's health, or from women's cancer. That's what I would have to do,
Mr. Chairman, and these are very difficult decisions. (1:16)
Taking into account all these discretionary funds, the total average annual amount that Health Canada could allocate to the fight against HIV and AIDS amounts to $42.2 million ($40.7 million + $1.5 million). However, the Canadian AIDS Society stated that the federal government is not even spending the Strategy's entire budget. In his testimony before the Sub-Committee, David Garmaise, Executive Director of the CAS, estimated that roughly $1.75 million was not spent in 1993-94. (2:6) The Sub-Committee requested further details on the subject from Health Canada. The information provided by the department is summarized in the following table:
(in millions of dollars)
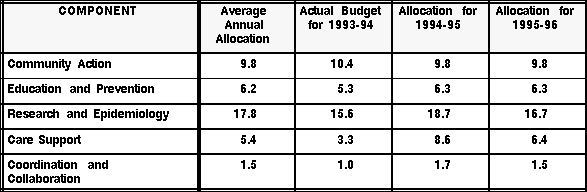

Source: According to information provided by Health Canada, February and May 1995.
In her letter of response, Kay Stanley, Assistant Deputy Minister, Health Programs and Services Branch (HPSB) (Health Canada), explained that Health Canada had deferred the Strategy's funds to the following year. The Strategy's total budget for 1993-94 thus amounted to $35.6 million, which means that $5.1 million ($40.7 million - $35.6 million) was not spent. Ms. Stanley stated that part of that amount, $4.2 million, was carried forward to the 1994-95 fiscal year. That meant that $0.9 million ($5.1 million - $4.2 million) was not spent at all. Furthermore, in 1993-94, Health Canada drew a total of $650,000 from the discretionary funds ($400,000 for the Vancouver Conference Organizing Committee and $250,000 for the Pacific AIDS Resource Centre in Vancouver). Consequently, roughly $850,000 ($1.5 million - $0.65 million) of the discretionary funds was not used. The two unspent amounts added together come to the unspent budget figure of $1.75 million estimated by the CAS ($0.9 million + $0.85 million).
Considering the $4.2 million deferred and the remaining discretionary funds of $1.1 million, the total budget of the National AIDS Strategy should have reached $46 million in 1994-95 ($40.7 million + $4.2 million + $1.1 million). In another letter to the Sub-Committee, Health Canada indicated that the 1994-95 budget had amounted to about $45 million and that the $4.2 million had effectively been reprofiled.
Furthermore, during her appearance, Kay Stanley (HPSB) hastened to reassure the Sub-Committee that the Strategy's budget was not cut for 1995-96 under last February's budget, which, in her view, was a major achievement given the current state of the government's finances.(24) The Minister of Health, for her part, stated that, in light of budget difficulties, all federal programs are subject to a review designed to determine whether those programs still meet the public's needs and whether they can justifiably be considered as a federal activity. The National AIDS Strategy will be subject to this review:
(24) Kay Stanley, Assistant Deputy Minister, Health Programs and Services Branch, Health Canada, Opening Remarks, 10 May 1995.
-
While HIV/AIDS continues to be a priority for this government, its funding has to
be examined like that of all other federal programs. I have already noted that it is
unlikely that new monies will be available for HIV/AIDS. Given our fiscal situation,
the challenge is not to spend every available dollar. It is to spend every dollar we
do spend wisely. (1:10)
The Minister also suggested that the budget could be misspent and that she will try to prevent this type of situation:
-
. . . I have observed since I've been Minister of Health . . . as you see the end of the
budget year come around . . . an attempt to spend all the money that's been
allocated for that year, with all kinds of projects, some very good, some not so
good. Frankly, for some I don't know whether it was such a great use of dollars.
. . . Knowing the fiscal and budget realities, I think the idea is not to rush to spend
all the dollars that are there but to make sure that we have all the best possible
programs and that we spend those dollars in the best possible way. (1:23-24)
It is unfortunate to see public funds misused when community AIDS organizations, for example, have no public funding when the demand for funding granted under the Strategy exceeds supply and when research expenditures are insufficient. The mid-term review provided under the Strategy's mandate is currently underway and should be completed by year-end. It is to be hoped that the review will help eliminate the misuse of funding earmarked for AIDS.
Most of the witnesses criticized the level of funding allocated by the federal government under the Strategy. For example, when Phase II of the Strategy was announced in 1993, the major national parties requested an average annual funding of at least $55.3 million. This funding level was also endorsed by the Ad Hoc Committee on AIDS.(25) In addition, the CAS emphasized that Strategy funding was established several years ago, but the budget was not adjusted for inflation and has not been reviewed on the basis of the growing needs of the community organizations that provide care to persons living with HIV/AIDS. The CAS explained that, because of advances in treatment, people with AIDS are living longer, but this also means they require care and support for a longer period of time. The organizations' client load is rising steadily, but since their budgets have been cut, some must reconsider the services they offer.(26) The Canadian Public Health Association (CPHA) expressed a similar view in its brief and reiterated the recommendation that funding for the National AIDS Strategy be increased to $55.35 million per year to meet the growing needs associated with the epidemic.(27)
(25) Canadian AIDS Society, Brief, 15 February 1995, p. 12.
(26) Ibid., p. 13.
(27) Canadian Public Health Association, Brief, 1 March 1995, p. 18.
David Garmaise (CAS) believes it is essential that an investment be made immediately in order to avoid enormous costs later:
-
The choice that Canada faces is not whether to spend money on AIDS but when
to spend it. We can allocate resources now to prevent the spread of HIV or we can
spend a lot more down the road to treat people who become HIV-positive. It costs
about $100,000 to treat a case of AIDS. To treat 1,000 cases of AIDS, then, would
cost $100 million. (2:6)
If we consider the 15,000 new AIDS cases expected over the next five years, these costs and the necessary investment in health and HIV/AIDS could radically destabilize public finances in the near future. Only a decisive, early investment can prevent us from postponing the bill for 10 or 15 years.
The representatives of the Coalition des organismes communautaires québécois de lutte contre le sida (COCQ-SIDA) also felt the federal government was not allocating enough money to AIDS and were indignant that Health Canada is not spending all of the budget. In the view of a number of community organizations, not to spend the allocated budget is contradictory and even dangerous: "How can we believe that our governments are concerned with the public's health when they boast of saving millions of dollars on health."(28) The Coalition further stated:
(28) Coalition des organismes communautaires québécois de lutte contre le sida, Brief, May 1995, p. 10.
-
The saving the federal government thinks it realized this year will multiply future
expenditures by a factor of 10. A government that deprives its citizens of health
care, a government that refuses to look at the situation square on, a government
that deprives citizens by failing to introduce prevention campaigns is, in our view,
an irresponsible government that does not heed the rights of its citizens.(29)
(29) Ibid., p. 12.
The Coalition recommended that monies "saved" between 1993 and 1995 be redeployed for the 1995-96 fiscal year.
The Canadian Nurses Association (CNA) also recognized that it is hard to make choices within an increasingly limited health budget: "No one likes to `trade off' or pit one disease against another, AIDS clients against cancer clients, the needs of children against the needs of the elderly."(30) The Association nevertheless maintained that the federal government must uphold its commitment and not reduce the Strategy's budget. It shared the CAS's opinion that the question is not whether money must be spent on AIDS, but when. The federal government would be ill-advised at this point to withhold AIDS funding.
(30) Canadian Nurses Association, Brief, February 1995, p. 9.
The Canadian Haemophilia Society (CHS) also emphasized that the federal government must not compromise the Strategy in the least and must provide adequate funding. This money must be allocated so as to facilitate cooperation rather than rivalry. Community groups and even the major partners often compete against each other for scarce resources. This situation works counter to the federal government's intention to foster partnerships. Durhane Wong-Rieger, CHS, said she was disappointed by the Health Minister's comments that there would not be any additional funds. (5:7)
Overall, witnesses agreed on the need for a sustained budget under the Strategy. Further, it is important that public resources be used properly. Therefore, the Sub-Committee recommends:
8. That Health Canada provide stable and continuing funding for the
National AIDS Strategy by spending the full amount of budgeted funds
during each fiscal year;
9. That the Minister of Health ensure that the funds earmarked for the National AIDS Strategy are properly managed.
B. Role of the Private Sector
When she appeared before the Sub-Committee, the Minister of Health indicated that the private sector was becoming increasingly involved in the fight against AIDS and she stated: "One of the major challenges that all partners must now meet is to find new ways to increase the private sector's role in this area."(31) The private sector consists of businesses, foundations or individuals who are willing to provide financial donations or support in the form of products, services, knowledge, or time and energy as a volunteer. A paper produced by the National AIDS Secretariat and submitted to the Sub-Committee contends that the goal is not to reduce federal government support:
(31) Hon. Diane Marleau, Minister of Health Canada, Speaking Notes, 8 February 1995, p. 4.
-
The concept of encouraging and discussing partnerships with the private sector
represents not a diminishing but rather a strengthening of government
commitment to national partners.(32)
(32) National AIDS Secretariat, Health Canada, National AIDS Strategy - Toward Building Private Sector Partnerships: A Discussion Paper, May 1994, p. 1.
In Canada, human resources, that is volunteers, constitute the most active private initiative in the fight against HIV/AIDS. It is estimated that some 10,000 volunteers get involved every year at the community level, contributing almost 1,000,000 hours for a value of $10 million.(33)
(33) Charles Fremes, Corporate and Public Affairs, Molson Breweries Limited, oral presentation before the Sub-Committee, 31 May 1995.
Canada has also introduced a number of initiatives in which the foundations or corporations are involved in the fight against AIDS. The Canadian Foundation for AIDS Research (CANFAR) is a national organization for the support of AIDS research which raises funds from the private sector. Roger Bullock, General Manager of CANFAR, said that contributions come from varied sources, including individuals (in some instances, in memory of someone or bequests representing part or all of an estate), charitable foundations, corporations (in particular, insurance companies and the banks) or through fund-raising events organized by the Foundation. (3:5) Since its inception, CANFAR has funded 97 research projects for a total value of $1.95 million. (3:6) Molson Breweries has also made a commitment to fight HIV and AIDS by introducing fund-raising projects. Molson contributes funding across Canada and, among other things, to Dancers For Life of Toronto benefits, the Kumbaya Music Festival and the "Walk for Life" in Montreal.
There are nevertheless barriers to private sector participation in the fight against AIDS in Canada. Many witnesses explained to Sub-Committee members that AIDS is a relatively new and still highly stigmatized disease. Most people do not yet consider themselves at risk and thus do not realize HIV's impact on society. Lastly, only a small portion of Canadians have been mobilized to take action to meet this challenge. It is therefore essential that the public be made aware of the impact of HIV and AIDS on society: approximately one in every 1,000 Canadians is infected with HIV and roughly one in 10,000 Canadians has AIDS. AIDS affects men, women and children in every social group. HIV creates needs that grow day by day as the epidemic progresses. "We're all living with AIDS."(34)
(34) Ibid.
Roger Bullock (CANFAR) indicated that the stigma still attached to HIV and AIDS makes it difficult to raise funds for research. He explained that companies are often looking for a return on their charitable investment and mere social responsibility or good will are generally not always sufficient arguments to convince them. (3:6) To convince the private sector and stimulate corporate participation, business- and marketing-related arguments should be used.
Witnesses also told members of the Sub-Committee that the lack of federal leadership has not convinced the private sector of the extent of AIDS. The National AIDS Secretariat admits that the onus is on the federal government, through its leadership and with the aid of national partners, to create the opportunities and the environment that will engender support and mobilize private sector involvement.(35) In addition, the Secretariat believes that further development and marketing skills to assist HIV/AIDS organizations in building and sustaining private sector support are required.(36) Therefore, the Sub-Committee recommends:
(35) Ibid.
(36) Ibid.
-
10. That the federal government, through Health Canada, develop a
communications strategy that publicly encourages initiatives by the
private sector (businesses and individuals) in the fight against
HIV/AIDS.
COMMUNITY ACTION
There was unanimous agreement throughout the Sub-Committee's hearings on one particular point: community involvement has always been one of the greatest weapons against AIDS. The Sub-Committee was told on a number of occasions that prevention, promotion and support efforts are most successful when made at the grassroots level. Community organizations provide the most effective services both for preventing the spread of HIV (education, promotion and prevention) and for helping those who are infected (care, treatment and support). Community groups know their public and their needs and clients feel comfortable turning to them.The effectiveness of community work may be explained in large part by the fundamentally important role played by volunteers. AIDS Calgary, for example, has only 10 employees, but in fact is 350 strong. The other 340 are volunteers giving time and services. (2:7) Similarly, COCQ-SIDA indicated that all the community organizations in Quebec together had only 150 permanent employees, but more than 2,500 volunteers. The Coalition tried to put a dollar figure on the work of all these people and estimated that, for a $667,800 investment by the federal government, nearly $5 million worth of time was generously donated by this group (based on a wage of $10 an hour).(37)
(37) Coalition des organismes communautaires québécois de lutte contre le sida, Brief, May 1995, p. 5.
The federal government encourages community participation. Through the AIDS Community Action Program (ACAP), Health Canada provides funds to community organizations and groups to enable them to conduct education, prevention, health promotion and community support and care activities. Tracey Donaldson, Manager of ACAP, Health Canada, stated the program's objectives:(38)
(38) Tracey Donaldson, Acting Manager, AIDS Community Action Program, Health Canada, Brief, 10 May 1995, p. 1-4.
-
1. To reach hard-to-reach groups whose activities place them at particular risk:
street-involved youth, gay youth, injection drug users and their sexual partners,
men who have sex with men, Aboriginal peoples, marginalized women, prison
populations and ethnocultural groups;
2. To support activities that increase the capacity of people living with HIV/AIDS to manage their condition, to delay the onset of symptoms through learning, to improve their access to services, treatment, care and social support;
3. To foster the creation of supportive social environments by eliminating barriers such as discrimination, poverty, fear and stigma associated with HIV/AIDS, which prevents certain groups from enjoying good health and from having access to the services they need;
4. To strengthen community development; and
5. To strengthen collaboration among organizations.
ACAP provides two types of funding: project funding and operational funding. Project funding is provided at the local, regional and national levels, that is to say to both community organizations operating at the local and regional levels and national non-governmental organizations (such as the five major partners). ACAP's operational funding is reserved for community groups only. Operational funding is used to fund the ongoing operation and daily operations of community action groups. It is used to maintain infrastructure, pay the salaries of community groups' staff and provide the working capital the organizations need to recruit, train and manage volunteers, develop their programs and deliver services. Project funding, on the other hand, is more temporary and intended for fairly specific activities. For example, Health Canada funded the Maggies - Toronto Self-Help Prostitutes Project, which is an information centre providing education and prevention services to sex trade workers in that city.(39) All proposals received by ACAP are subject to an external peer review process and pre-selected proposals then undergo departmental review.
(39) Health Canada, Briefing Notes: Women and AIDS (30 November 1994), Briefing Book for the Sub-Committee on HIV/AIDS, 14 December 1994.
When Phase II of the National AIDS Strategy was announced, the federal government stated that an average annual budget of $7.5 million would be allocated to ACAP.(40) In 1993-94, the program's funding was increased to $8.1 million as a result of a reallocation of funds from the care, treatment and support component's budget. Financial data for 1994-95 are not yet available. Furthermore, Barbara Jones of the AIDS Education and Prevention Unit, Health Canada, indicated that the total budget set aside for 1995-96 would be $7.5 million.(41)
(40) Health Canada, National AIDS Strategy: Funding, Fact Sheet, May 1994.
(41) Barbara Jones, Chief, AIDS Education and Prevention Unit, Health Canada, Presentation to the Sub-Committee, 10 May 1995.
The national organizations and community groups that appeared before the Sub-Committee all underscored ACAP's importance for the communities. The community organizations very much need the project and operational funding they receive under ACAP. As the CAS noted, a number of those organizations in certain provinces receive no money from any other public funding source.(42) The CAS also emphasized that Health Canada has shown considerable generosity toward the national organizations in the area of project funding.(43)
(42) Canadian AIDS Society, Brief, 15 February 1995, p. 14-15.
(43) Ibid., p. 7.
However, witnesses felt that funding for community initiatives was inadequate. They explained that the community organizations' workload is increasing proportionally as the epidemic spreads, but not only has their funding not increased proportionally, it has, on the contrary, declined. For example, the Sub-Committee was informed that grants to AIDS Nova Scotia and the Nova Scotia Persons with AIDS Coalition were cut by $20,000 and $10,000 respectively in 1994-95, even though AIDS Nova Scotia's clientele increased by 82% and that of the Nova Scotia Persons with AIDS Coalition increased by 380% in one year. The two organizations decided to merge in 1995-96. However, they were informed that their funding would be further cut by between $20,000 and $30,000.(44) "If we are going to win the war against AIDS," David Garmaise told the Sub-Committee, "community groups need additional funding." The Sub-Committee also learned that the organizations' clientele has diversified. Ten years ago, it consisted almost solely of gay men; today, however, it also includes women, and Aboriginal peoples. The community programs need to be tailored to this new clientele. The organizations must respond to ever more numerous demands with limited budgets. The Canadian AIDS Society pointed out that some community organizations received no funding, but are in great need of it. There has been no coordinated response between the Strategy and front-line workers, as a result of which the organizations must convert money intended for core funding to project funding when no money is available elsewhere. The Society indicated that the instability and short-term nature of the funding greatly limits the organizations' planning.
(44) Ibid., p. 14.
The Canadian AIDS Society also informed the Sub-Committee that the federal government intends to gradually replace operational funding with the project funding it provides under ACAP. According to the CAS, this move is not at all responsive to the needs of the HIV/AIDS organizations working on the front lines.(45) On this point, COCQ-SIDA indicated that it would like project funding to be reduced in favour of operational funding so that community infrastructures can be maintained and reinforced and more generic projects carried out in cooperation with other partners. More specifically, it recommended that monies allocated to project fundings be cut in favour of more support for operation and suggested a transfer of some 25% of funding.(46) Lastly, COCQ-SIDA also mentioned that ACAP project funding does not make it possible to carry out short-term projects. However, the Coalition felt that it could be helpful to carry out new, more long-term initiatives, particularly in education and prevention.
(45) Ibid. , p. 14.
(46) Coalition des organismes communautaires québécois de lutte contre le sida, Brief, May 1995, p. 11.
The Canadian Nurses Association (CNA) also underscored the importance of recognizing the work done by community organizations. The Association recommends that community-based programs should be supported and allowed the flexibility needed so that clients can determine the most appropriate programming to meet their needs. According to the CNA, the federal government can make community care programs more effective.(47)
(47) Canadian Nurses Association, Brief, February 1995, p. 12.
Lastly, the Canadian Haemophilia Society (CHS) also shares the view that, through ACAP, the National AIDS Strategy has made it possible to establish a network of community organizations whose work is formidable and highly effective. In the CHS brief, Durhane Wong-Rieger stated: ". . . I am truly amazed at their effectiveness and impact. No government organization, no business or industry could match their ratio of output for dollar invested."(48) Like most of the other witnesses, Ms. Wong-Rieger contended that the community care network needs reinforcement and she recognized that that would require a considerable amount of funding.
(48) Durhane Wong-Rieger, President, Canadian Haemophilia Society, Brief, 15 March 1995, p. 5.
In order to be able to respond to the epidemic's increasing needs, community organizations must have stable funding over a long period. In addition, Health Canada should provide more flexibility over operational funding and project funding. Therefore, the Sub-Committee recommends:
11. That Health Canada commit itself to maintaining the level of funding for
the AIDS Community Action Program;
12. That Health Canada provide stable, long-term funding for community organizations involved in the fight against HIV/AIDS;
13. That Health Canada re-address the issue of core vs. project funding and establish funding mechanisms that are agreeable to all parties.
EDUCATION AND PREVENTION
Since no AIDS vaccine or effective antiviral agent exist, education is the only tool we have to prevent HIV transmission. The National AIDS Strategy contains an education and prevention component. More particularly, Barbara Jones of the AIDS Education and Prevention Unit, explained to the Sub-Committee that this component consists of five specific functional areas:(49)
(49) Barbara Jones, Chief, AIDS Education and Prevention Unit, Health Canada, Brief, 10 May 1995, p. 4-9.
-
1. Under the National Health Research and Development Program (NHRDP), the
Unit funds extramural research on prevention as such and on attitudes and
behaviour;
2. The Unit synthesizes information on education and prevention efforts and provides financial support for community-level program evaluation;
3. The Unit funds the organizations and provinces that have developed innovative prevention programs;
4. The Unit undertakes specific initiatives with other departments or government agencies (e.g. Correctional Service Canada) and trains customs and immigration officers; and
5. Lastly, the Unit provides core and operational funding for projects initiated by the five major national partners.
When Phase II of the Strategy was announced, the federal government stated that it would allocate an average of $6.2 million to education and prevention initiatives.(50)
Ms. Jones indicated that the funding granted in 1995 and 1996 would be $1.95 million for extramural research, information synthesis and the development of innovative programs, $600,000 for interdepartmental initiatives and $2.8 million for funding of national non-governmental organizations.(51)
(50) Health Canada, National AIDS Strategy: Funding, Fact Sheet, May 1994.
(51) Barbara Jones, Chief, AIDS Education and Prevention Unit, Health Canada, Presentation to the Sub-Committee, 10 May 1995.
In Canada, the Canadian Public Health Association (CPHA) also plays an important role in the prevention of AIDS through its National AIDS Program. The mission of the program, which was introduced in 1986 with Health Canada's cooperation, is "to provide national leadership in supporting the development and delivery of programs and public health policies which prevent HIV transmission and support the care of those affected by HIV/AIDS."(52) The program is guided by principles of cultural appropriateness, accessibility, partnership, non-judgmental programming and social equity. CPHA's National AIDS Program consists of four main areas of activity:
(52) Canadian Public Health Association, Brief, 1 March 1995, p. 3.
-
i. educational activities (media campaigns, seminars, educational resources and
consultation services);
ii. public policy (development of an orientation paper for health professionals, public health authorities and public decision-makers);
iii. communications (bilingual national newsletter devoted to AIDS education and a monograph series); and
iv. the National AIDS Clearinghouse. The Clearinghouse provides documents for use both in and outside Canada, including papers, brochures and videos. It also provides information reference services. More than 89% of the Clearinghouse's clientele considers the services good or excellent.
During the Sub-Committee's hearings, witnesses stressed the benefits of the federal government's education and prevention efforts. The CAS, in particular, indicated that Health Canada had helped produce major reference works on the nutrition of persons living with HIV/AIDS or on program evaluation, as well as studies on ethnocultural communities.(53) The CAS also pointed to progress made in federal immigration policies. Lastly, the Society noted the introduction of a condom distribution program in federal penitentiaries and welcomed Correctional Service Canada's decision to pilot test bleach distribution, anonymous HIV-testing and peer counselling.(54)
(53) Canadian AIDS Society, Brief, 15 February 1995, p. 8.
Blank Line
(54) Ibid., p. 7.
A. Barriers to Effectiveness of Prevention Measures
Despite progress made in AIDS education and prevention, epidemiological studies have shown that the number of cases is unfortunately still increasing. According to witnesses, there are barriers to the effectiveness of awareness and prevention measures. The CAS argued that "censorship compromises effectiveness." It stated that we must abolish restrictions on the use of explicit language, promote access of educators to schools, residential facilities and penitentiaries and raise limits on subjects that can be discussed during visits to those institutions.(55)
(55) Ibid., p. 20.
Ron de Burger, Director, National AIDS Program (CPHA) emphasized that one major barrier to the effectiveness of education and prevention programs is still attitude. In his view, too many Canadians think it is not necessary to talk about sexual health. What is more, many do not feel concerned and still believe they are invulnerable:
-
. . . often people are not prepared to listen to the message until they see the
effects of the problem. That's certainly been the case with HIV and AIDS, as it has
been with other communicable disease entities. Many people believe they are
invulnerable, that it's not going to happen to them and that somehow they are
going to remain unaffected in any particular respect. (4:13)
Dr. Brent Kvern of the College of Family Physicians of Canada (CFPC) stressed the need to change attitudes toward both sexual orientation and lifestyle. Regarding injection drug use, for example, he said:
-
We all know it's not injection drug use per se that puts you at risk but the activities
associated with injection drug use. People don't necessarily choose to become
injection drug users, that lifestyle. There might be other issues going on as an
illness that needs to be cared for, but in the meantime it needs to be treated in the
safest way possible. (5:30)
A certain number of witnesses stressed that education, promotion and prevention programs are not being evaluated, as a result of which we do not really know whether they are effective. Ms. Wong-Rieger of CHS, for example, stated:
-
We have not articulated what it is we want to achieve and what we can expect to
achieve. We do not make sure our programs and activities are lined up to ensure
we can achieve those. . . We don't go back to modify and redefine. (5:14)
The lack of evaluation also limits efforts to improve existing programs. What is more, witnesses said that the effectiveness of education and prevention measures is greatly compromised by the fact that the programs funded under the Strategy are short-term and not coordinated. The CAS indicated in its brief that the federal government was not spending enough time, energy and money on prevention campaigns to ensure their long-term effectiveness. In their view, current prevention activities are, for the most part, sporadic and cosmetic.(56) The CAS also indicated that every organization is working in isolation, with no master plan and no national strategy. Since prevention activities are not coordinated, there is nothing "to ensure that groups providing education locally are not reinventing the wheel." In the CAS's view, the organizations should be afforded more opportunities to meet each other and share their experiences, and the federal government can play a role in this regard.(57) The Canadian Nurses Association, for its part, stated that prevention activities are effective and offer many benefits when sustained over time and coordinated across government and communities. According to CNA, the federal government must take a strong leadership role in coordinating efforts.(58) Similarly, Ron de Burger (CPHA) said that a sustained long-term commitment is necessary in order to bring about behaviourial changes and improvements. (4:13) Durhane Wong-Rieger of CHS shared that view:
(56) Ibid., p. 24.
(57) Ibid.
(58) Canadian Nurses Association, Brief, February 1995, p. 5.
-
We know the only thing that's effective in terms of changing current practices with
regard to protection from HIV and AIDS is societal norms. As a psychologist, I
think I would be remiss in not pointing out that changing societal norms is not a
short-term process. It's not a process that's going to happen without a great deal
of intensive effort.
We've seen some changes. We've seen some progress. The worst possible thing that could happen now is that we would let up on that effort whatsoever. More than anything else, we have to be evaluation- and outcome-driven. (5:14)
Therefore, the Sub-Committee recommends:
- 14. That Health Canada conduct an evaluation of its education and
prevention programs in order to determine their impact on Canadians'
attitudes and behaviours and make any changes to these programs that
are found to be necessary;
15. That the education and prevention programs developed under the National AIDS Strategy be supported over the long term in order to lay special emphasis on behavioural changes.
B. General or Specific Prevention?
During the hearings, participants wondered whether it would be preferable to work toward general prevention aimed at everyone or rather to target the most vulnerable groups. Some felt that, if only high-risk groups were targeted, we would lose the broader view of the issue, that AIDS would no longer be considered as important and we would not be protecting the next generation because we would only be interested in specific groups. Others, on the other hand, claimed that a general approach is not effective where there are active and dynamic epidemics among target groups such as injection drug users, young homosexual men, minority women living in poverty. According to the CAS, wide-ranging campaigns have limited appeal because they are not specific enough to the groups for which they are in fact intended. CPHA, on the other hand, would like to see more national awareness campaigns. The right balance must therefore be struck between national and local campaigns and between programs for the general public or those aimed at specific groups.A number of witnesses nevertheless placed their emphasis on the various prevention and education measures aimed at particular groups. CPHA, for example, stressed the importance of developing programs for young people. The Association stated that many young people continue to ignore AIDS prevention messages and that they have not abandoned their high-risk sexual behaviours. In CPHA's estimate, some 350,000 young people become teenagers every year. They need basic AIDS information and they also need to acquire more skills and be given more supervision before they are able to make the right decisions. Effective prevention programs are essential to protect our youth. CPHA said it was in favour of the mandatory inclusion of sexual health programs in the schools and that it was also in favour of establishing sexual health clinics in the secondary schools, in particular to encourage behaviours that reduce the risk of HIV transmission. Even though teenagers have basic information on HIV transmission, that unfortunately does not mean their behaviour is any safer. To promote behaviourial change, CPHA suggested having young people take part in the design and implementation of AIDS education programs.(59)
(59) Canadian Public Health Association, Brief, 1 March 1995, p. 10-11.
Eleanor Ross, President of the CNA, said that preventive action must be taken before the problem arises. Prevention must be done with young people before they find themselves in the street, like the homeless and drug addicts. They must be made to understand in advance what safe sex and drug abuse are all about. (4:29) CNA believes that HIV/AIDS education for children should begin in the elementary schools and be provided in a frank and explicit manner. According to the Association, education programs on television are also a good way to disseminate information for children and adolescents. It referred, in particular to the CBC English-language program "AIDS Scare/AIDS Care," in which popular youth personalities tell teens, in their own language, about the risks of HIV/AIDS, how it is spread and how to protect themselves. The Association emphasized that the earlier education begins, the more effectively sound rules for living are learned.(60)
(60) Canadian Nurses Association, Brief, February 1995, p. 5.
With regard to the education of young children, the Honourable Judy Erola, President of the Pharmaceutical Manufacturers' Association of Canada (PMAC), indicated that her Association funded the publication, in both official languages, of a storybook entitled "Come Sit By Me." The book was written for children 4 to 8 years of age and their teachers. Ms. Erola said that it had been distributed to all doctors' offices across the country. (6:19) Some members of the Sub-Committee found the storybook interesting and expressed the view that it would perhaps be appropriate to distribute it to all the primary schools across Canada.
Some witnesses expressed concern about the increasing numbers of new HIV infections among women and children. It appears that the danger women run of being infected by HIV is related to their own high-risk behaviour or to that of their sexual partners. Lack of autonomy, submission, violence and poverty prevent women from protecting themselves against HIV.(61) In addition, women can transmit the virus to their children. According to COCQ-SIDA, the spread of HIV among women has given rise to "a need that remains unfulfilled for lack of additional financial resources."(62) Both the CNA and the CMA asked that greater attention be given to prevention and promotion programs intended for women and children.(63)
(61) Health Canada, Briefing Notes: AIDS and Women (30 November 1994), Briefing Book for the Sub-Committee on HIV/AIDS, 14 December 1994.
(62) Coalition des organismes communautaires québécois de lutte contre le sida, Brief, May 1995, p. 8.
(63) Canadian Nurses Association, Brief, February 1995, p. 5; Canadian Medical Association, Speech to the Sub-Committee, 28 March 1995, p. 3.
Many witnesses said they were concerned by HIV infection rates among drug users. The epidemiological data provided to the Sub-Committee show that HIV transmission among injectable drug users is increasing in spectacular fashion in Canada and that shared needles appear to be the most effective method of spreading HIV. Most witnesses said they were in favour of needle exchange programs.
The Sub-Committee was also told, "The situation in the prisons is like a ticking time bomb."(64) CPHA explained that there is a particular need for prevention in prisons:
(64) Canadian AIDS Society, Brief, 15 February 1995, p. 18.
-
The proportion of the incarcerated population which uses injection drugs is much
higher than in the general population. The restrictions of prison life also make it
much more difficult to obtain the condoms or clean needles to reduce risk in
sexual encounters and in drug use. Depression, lack of self-esteem and the
inability to see a future for oneself are common among the prison population and
decrease the motivation to protect one's life and future. These conditions make
for an environment in which HIV can spread quickly. After living in an environment
with a much increased risk of exposure, prisoners make parole or finish their
sentences and return, possibly infected, to their home communities.(65)
(65) Canadian Public Health Association, Brief, 1 March 1995, p. 10.
In his brief, Dr. Norbert Gilmore, who chaired the Expert Committee on AIDS and Prisons (1992-1994), expressed a similar view.(66) According to CPHA, the Expert Committee's final report constitutes an excellent point of departure for responding to the specific needs of prison staff and inmates and the Association suggested implementing the 88 recommendations contained in that report.(67)
(66) Dr. Norbert Gilmore, Chair, Expert Committee on AIDS and Prisons, Brief, 3 May 1995.
(67) Canadian Public Health Association, Brief, 1 March 1995, p. 11.
In his appearance before the Sub-Committee, John Edwards, Commissioner of the Correctional Service of Canada (CSC), indicated that CSC largely supported the recommendations by the Expert Committee on AIDS and Prisons, but that three specific suggestions had been rejected: remove current prohibitions against consensual sexual activity between inmates, provide methadone maintenance programs for opiate-dependent inmates and establish a needle exchange program. As an alternative to needle exchange, CSC agreed to pilot test a bleach distribution program for sterilizing syringes used for drugs and tattoos.(68)
(68) John Edwards, Commissioner of the Correctional Service of Canada, Brief, 3 May 1995, p. 10.
The Canadian AIDS Society feels that prevention measures introduced in the prison system are piecemeal and do not go nearly far enough. According to the Society, Correctional Services Canada is not responding adequately to the epidemic. Inmates' rights are being violated because they do not have the same means to protect themselves and to take care of their health as the general public.(69)
(69) Canadian AIDS Society, Brief, 15 February 1995, p. 17-18.
The Sub-Committee was also informed that, as the epidemic in Canada evolves, the Aboriginal populations are at growing risk for HIV infection and AIDS. Janice Hopkins, Director General of the Indian and Northern Health Services, Health Canada, discussed the risks of the spread of HIV/AIDS in urban centres as well as on reserves and in remote regions:
-
Transmission of HIV among Aboriginal people is facilitated by high rates of other
STD's; high rates of substance abuse including injection drug use; and other
health status issues. These risks are compounded by the over-representation of
Aboriginal people in inner-city populations where rates of HIV and associated risk
behaviours are relatively high. High mobility between inner-cities and rural and
reserve communities brings the risk of HIV to even the most remote Aboriginal
communities.(70)
(70) Janice Hopkins, Director General, Medical Services Branch, Health Canada, Speaking Notes, 10 May 1995, p. 2.
In her brief, Ms. Hopkins mentioned that condoms are available in all health care institutions serving the department's Aboriginal clientele and that needle exchange services are available on demand.(71) However, a report submitted to the Sub-Committee indicates that HIV prevention and education initiatives are still not widely available in all reserve communities, and there is resistance to implementing them in some communities. That report also emphasized that the activities in place tend to focus on general awareness of HIV/AIDS, that prevention and education should be better targeted and that greater emphasis should be placed on risk behaviour and attitudes.(72)
(71) Ibid., p. 9.
(72) Health Canada, Interjurisdictional Coordination on HIV/AIDS and Aboriginal Populations: Issues and Approaches, January 1995, p. 4.
Lastly, COCQ-SIDA noted that the homosexual community is still the most affected by HIV/AIDS. The Coalition mentioned that, although a significant increase in safe sexual practices has been observed among gays, there has nevertheless been a rise in the incidence of risk behaviours among homosexuals under 25 years of age. It recommended that a national campaign for the prevention of HIV infection be organized among the homosexual community.(73) Once again, it was argued that prevention efforts must be made on a continuing basis in order to sustain safe sexual behaviours:
(73) Coalition des organismes communautaires québécois de lutte contre le sida, Brief, May 1995, p. 7, 8 and 11.
-
. . . studies show that even when new behaviours have been adopted, there is a
need for ongoing support in order to sustain the change. Individuals become
depressed at the loss of friends and loved ones, which has been particularly
acute in the gay community. They may lose their motivation when the subject
becomes too familiar and people stop talking about safer sex. They may even
develop unconscious feelings of invulnerability when they "get away with" risky
behaviours. Being in a close relationship may give a false sense of security.
Health promotion campaigns and peer support methods must be more fully
utilized to sustain healthy behaviour change.(74)
(74) Canadian Public Health Association, Brief, 1 March 1995, p. 10.
In short, we must not stop our efforts and imagine that the message has been understood until the number of persons infected with HIV has truly fallen. Awareness efforts must be continued and sustained as far as possible. In addition, education and prevention activities to stem the AIDS epidemic must be carried out on all fronts - local, provincial, territorial and national - on the basis of partnerships with the participation of the people concerned. Therefore, the Sub-Committee recommends:
16. That Health Canada, in cooperation with the other stakeholders in the
fight against HIV/AIDS, evaluate current programs for raising
awareness of HIV/AIDS and prevention measures designed for target
groups in order to improve their effectiveness;
17. That Health Canada, in cooperation with other stakeholders in the fight against HIV/AIDS, decide together on the most effective allocation of funds between broad campaigns and specific programs.
CARE, TREATMENT AND SUPPORT
One of the objectives of Phase II of the National AIDS Strategy is to enhance the capabilities of HIV caregivers to provide better quality care and treatment. At Health Canada, this objective falls to the AIDS Care, Treatment and Support Unit, whose mandate is "to facilitate and support projects and activities which will improve the quality of life of people with HIV/AIDS."(75) The term "caregivers" covers all professional and non-professional care providers, including physicians, nurses, social workers, volunteers working within community organizations, families and friends of persons living with HIV/AIDS.
(75) Health Canada, National AIDS Strategy - Phase II: Progress Report 1993-1994, Ottawa, 1994, p. 15.
In his brief to the Sub-Committee, Robert Shearer, Acting Chief, AIDS Care, Treatment and Support Unit, explained that the Unit supports five particular types of activity.(76) First, the Unit provides financial support for the education and training of caregivers. For example, it finances the publication of guides and guidelines, takes part in improving teaching programs in universities and supports development programs. The Unit has funded the development and publication of a guide for AIDS patient care by the College of Family Physicians of Canada, as well as a document prepared by the Canadian Nurses Association for its members. The Sub-Committee received a copy of these documents. A budget of $1 million will be allocated to this activity in 1995-96.
(76) Robert Shearer, Brief, 10 May 1995.
Second, the Unit funds research projects on psychosocial support and promotion of quality of life. For example, the Unit provides funding to the Canadian AIDS Society, which, with other partners, has begun a research project on the specific housing needs of persons living with HIV/AIDS. The budget allocated for the 1995-96 fiscal year for this activity will be $1.6 million.
Third, the Unit promotes the development of new models of care delivery and the assessment of effectiveness of existing programs. For example, COCQ-SIDA is currently developing a model designed to assess the services it provides. It is relying on this kind of contribution in order to complete its model.(77) The Unit also finances research on the economic aspects of HIV/AIDS. This research work, which will take roughly three years, is being conducted by the Canadian Policy Research Network. Health Canada plans to allocate a total of $700,000 to this activity in 1995-96.
(77) Coalition des organismes communautaires québécois de lutte contre le sida, Brief, May 1995, p. 10.
Fourth, the Unit also funds the AIDS Treatment Information Service. People with HIV/AIDS and those who provide care to them have exercised considerable pressure and are welcoming this new service. The service will make available all reliable and up-to-date information on medical treatments and other therapies that will help patients and their caregivers make informed decisions on therapeutic options. In 1995-96, the federal government will contribute some $2 million to this activity.
Fifth, the Unit is responsible for funding the Canadian HIV Clinical Trials Network, which will receive a budget of $2.9 million in 1995-96. The Clinical Trials Network is discussed in greater detail below.
In total, the AIDS Care, Treatment and Support Unit will pay out more than $8 million in grants and contributions as part of these activities. All proposals received undergo a review, which consists of an internal assessment by Health Canada and an external peer review. In his brief, Mr. Shearer stressed the Unit's multidisciplinary approach because care, treatment and support for persons with HIV/AIDS bring into play a wide range of people and require various types of services.
In her testimony, Eleanor Ross, CNA, explained that the various types of care that persons living with HIV/AIDS need depend on how the illness progresses. (4:25) First, there is intermediate support provided at home or in the community. This may be support that enables the patient to continue working or to stay in bed at home and receive home care. Acute care is highly technological and intensive care that is provided in the hospitals. Lastly, there is palliative care, the purpose of which is to protect the value of existence, to preserve its meaning and to improve quality of life. This care is provided to the dying and their relatives when it is no longer appropriate to prolong life or administer curative treatment. Patients' pain can be relieved and their other physical, emotional and spiritual needs met.
The representatives of the CNA and other witnesses who appeared before the Sub-Committee strongly recommended community services and home care because, they said, the best care is that provided as near as possible to the patient. In its brief, the CNA deplored the fact that, as part of the reform of the health care system, cuts in active care are not offset by community support services. According to the Association, there are not enough intermediate care services, such as semi-supervised structures where health, food and social assistance services can be found during the day. This type of care is often necessary when the spouse must go to work, but cannot leave the patient alone all day. CNA also stated that education and support for health care providers and informal caregivers working with HIV/AIDS clients remains key to providing quality care.)(78) It further recommended that governments at all levels ensure a continuum of care for HIV/AIDS clients including acute (acute, not active), intermediate and palliative care.(79)
(78) Canadian Nurses Association, Brief, February 1995, p. 7.
(79) Ibid., p. 12.
The Canadian AIDS Society(80) seems to have a similar opinion regarding community care and home care. In its brief, it also emphasized the need to establish more community-based palliative care hospices like Casey House in Toronto. It considers that, even though these strategies will require an initial investment, they will result in significant savings to the health care system in the long run. The CAS further believes that, although responsibility for the delivery of health care falls in large part to the provincial governments, the federal government has a role to play in this area. For example, the Society suggested that Health Canada can fund pilot projects and play a leadership role in bringing the provinces and other players together to search for innovative responses.
(80) Canadian AIDS Society, Brief, February 1995, p. 18.
When it appeared before the Sub-Committee, the CMA discussed the very important role physicians play in the fight against AIDS. The physician is the patient's primary link to HIV/AIDS health care. In addition, the doctor may often meet the needs of partners, families and advocates by means of information and supportive discussions. However, the Association and a number of other witnesses admitted that there are barriers to providing high quality care. Furthermore, HIV/AIDS is a complex condition that is difficult to understand and treat. On this point, Dr. David Walters of the CMA stated:
-
To answer questions from people and provide care, often when there are limited
treatments and no clear answers, is an extremely challenging and stressful task
for physicians who work in this field. (6:6)
Dr. Brent Kvern (CFPC) indicated that certain physicians feel they do not know the illness well enough to treat it properly. He also mentioned that the faculties of medicine "don't teach issues of sexual health" and that physicians are not well prepared and are reluctant to ask questions that may seem "embarrassing." (5:25)
Furthermore, it appears that their method of charging a fee for service also discourages physicians from treating HIV/AIDS patients. Dr. Kvern (CFPC) stated the following:
-
I think one of them is that certainly there's a financial disincentive to care for
people who have HIV disease. The way that many physicians are reimbursed,
simply stated, is that time is money. It takes time to care for people who are sick. It
takes time to care for people who have issues for which they may need
counselling or who may need to spend a lot of time talking about them. That's one
aspect. (5:25)
Lastly, the Sub-Committee was told on a number of occasions that there is a problem of attitude, prejudice and homophobia with respect to certain groups, which greatly affects the quality of care. On this point, the representatives of the CNA and CFPC explained that physicians, like society, constitute a heterogeneous group. There are still physicians who have not yet confronted their homophobia or their inability to treat intravenous drug users, to accept people for what they are and to help them. HIV/AIDS affects a population with which physicians are not always comfortable. There are individuals with various risky behaviours that physicians are not always able to care for without being judgmental. For example, Dr. Walters (CMA) told the Sub-Committee:
-
. . . it's obvious that as a society we have to deal with an attitude change, and we
have tried to deal with that attitude change. The attitude changes may involve
being homophobic, having problems dealing with drug users and other
disadvantaged people, or how to work with Aboriginal peoples and their cultural
approach to things. It is a major educational effort that all health care workers
need to respond to. (6:16)
These road blocks to the quality of care explain, in large part, the problem of the shortage of physicians specialized in the treatment of HIV/AIDS. In its brief, the CAS emphasized that it is hard to attract new physicians to the world of HIV and that the physicians specialized in this field are exhausted. In light of the increase in the rate of seroprevalence, the CAS believes that the current shortage may well cause problems in the near future if the situation is not immediately corrected:
-
Is Canada prepared to handle the 15,000 new AIDS cases expected over the next
five years? In this era of cost-cutting and downsizing, we see serious challenges
ahead.(81)
(81) Canadian AIDS Society, Brief, February 1995, p. 18.
For these reasons, a number of witnesses felt it was imperative that we improve education programs and provide continuing training programs for health professionals in order to (1) increase knowledge of HIV and AIDS, (2) change attitudes so as to develop the trust and ease needed to care for people living with HIV/AIDS, and (3) increase the number of physicians specialized in the field.
Efforts are already being made through funding from Health Canada. For example, the CMA has published the third version of its publication entitled, Human Immunodeficiency Virus Serologic Testing Counselling Guideline. (6:5) This publication is intended for physicians and other health professionals and contains specific guidelines regarding counselling and the information that should be given to patients regarding serologic testing. These guidelines are followed by health professionals treating HIV/AIDS patients. The College of Family Physicians of Canada has also developed A Comprehensive Guide for the Care of Persons with HIV Disease, which contains a module on the treatment of adults and a second on the treatment of children (which should be available shortly).(82) The College has also developed a training program for family physicians on the subject of HIV/AIDS infection. As part of this program, seminars on screening tests and counselling were given across the country. The College then developed teaching material - in particular slides and videos - and more information tours were conducted, with emphasis on the clinical skills needed to treat HIV infection confidently. Lastly, the College drew on the concept of training trainers: more experienced physicians who advise those with less experience in the care of HIV/AIDS patients. In 1992, CNA also published a guide on nursing care entitled, HIV/AIDS Education for Nurses: Practice Issues and Curriculum Guidelines, and it plans to conduct a survey of nursing schools in the near future to determine whether courses on HIV/AIDS should be included in their curricula.(83)
(82) College of Family Physicians of Canada, Brief, March 1995, p. 5.
(83) Canadian Nurses Association, Brief, February 1995, p. 3.
Lastly, witnesses indicated to the Sub-Committee that, in order to ensure the quality of care, medical staff must provide information on the care and treatment of HIV/AIDS patients so that they may come to an informed decision. James Kreppner of the Canadian Haemophilia Society feels that physicians are patronizing at times:
-
. . . sometimes doctors don't share test results with you because they think it's not
in your best interests for you to know, or they don't tell you the full risks and
problems that might be inherent in a certain treatment . . . That kind of behaviour
is unacceptable. Consumers have to be informed. They have to be in a position
where they can make informed decisions. (5:17)
Mr. Kreppner also told Sub-Committee members:
-
What we're seeing in the experience with blood . . . Part of the problem with that
old tragedy is that people weren't informed. People didn't know what risks they
were taking when they took blood. That can be carried over to the HIV experience
in general and the AIDS experience in general. Let's make sure we don't make the
same mistakes in HIV treatment. (5:17)
It is to be hoped that the National AIDS Treatment Information Service will provide the information necessary for informed decision-making both for the patient and for the caregiver.(84) Furthermore, all witnesses agreed on the need for adequate training as part of the courses provided by educational institutions and continuing training to encourage participation by professionals in the treatment of HIV/AIDS, to provide them with up-to-date information and to instruct them in new treatments. Therefore, the Sub-Committee recommends:
-
18. That, under the National AIDS Strategy, Health Canada continue to
support the professional medical associations in their efforts to update
and make available information about care to be provided to persons
with HIV/AIDS.
(84) In her opening remarks on 10 May 1995, Kay Stanley, Assistant Deputy Minister responsible for overseeing the implementation of Phase II of the Strategy, indicated that the Treasury Board had recently approved Health Canada's request to negotiate a three-year agreement to fund the AIDS Treatment Information Service. This project will be administered by the Community AIDS Treatment Information Exchange (CATIE) in Toronto.
RESEARCH
HIV/AIDS research falls essentially into two large categories, clinical trials and basic research. Also of importance are research in social science, HIV/AIDS epidemiology (the study of the incidence, distribution, control and prevention of this disease), and applied research which has tended towards the development of commercial technologies to detect and follow disease progression. Basic research focuses on trying to elucidate the fundamental basis of HIV disease and the opportunistic infections that accompany it. This includes expanding our knowledge of human immunology and physiology, and achieving an understanding of the pathogenesis, viral physiology and molecular biology of HIV. Comprehension of the make-up of HIV and how it attacks the immune system allows scientists to design drugs to target and block viral functions. These agents are tested in the laboratory to determine if they are effective against HIV and they are tested in animals to get an estimate of drug toxicity. After this point, clinical trials are designed for the purpose of testing the effectiveness and toxicity of the agent in humans. Clinical trials, which can cost millions of dollars, may be ongoing for years and involve thousands of test volunteers.Under the NAS, $17.8 million is allocated for all types of research and epidemiological monitoring. The $17.8 million per annum is broken down into $2.9 million for the Canadian HIV Trials Network (CTN), $1.5 million for research and development activities in the area of social and economic support, $5.5 million to the National Health Research and Development Program (NHRDP) to fund extramural research, and the balance supports intramural Health Canada research activities such as epidemiological monitoring, and the various research activities of the Bureau of HIV/AIDS Laboratories and Research. Further, the Medical Research Council (MRC) of Canada spends $2 million on HIV/AIDS research.
A. Clinical Trials
Clinical trials may have up to four phases. After the drug has undergone animal testing, it is ready to be tested in a phase I trial in humans to determine if the agent is safe. Test participants all receive the same known drug but in differing quantities so as to reveal dose-related toxicity. At this time, some early information on how well the drug works may be gleaned. Phase I trials last only two to three months and usually involve less than 100 people.If the drug appears to be safe enough, a phase II trial is conducted to more carefully study side effects and to determine if the agent has beneficial effects, such as raising T4 cell counts, or clearing up an infection. Phase II can last a few weeks to a few months and again usually involves less than 100 people.
Phase III trials are initiated if the drug has been observed to be effective. At this phase, usually hundreds and sometimes thousands of people are given the drug to see if it works for everyone and if it causes problems over a long period of time. Researchers look for rare side effects which are only seen in a few people or after a few years. A drug must go through all three phases of testing before the Drugs Directorate of Health Canada will consider granting it market approval.
Phase IV trials, or post-marketing trials, are not always conducted but they are becoming more important now that some drugs are approved earlier than in the past. They allow for more testing over a longer period of time, to see if any problems develop over the long term.(85)
(85) AIDS Action Now!, AIDS and HIV Drug Trials in Canada - What You Need to Know, 3rd Edition, Toronto, April 1993, p. 3-4.
In Canada, virtually all clinical trials of anti-HIV therapies and drugs to control HIV-related opportunistic infections are conducted by the CTN, which is a partnership committed to developing treatments, vaccines and a cure for HIV disease and AIDS through the conduct of scientifically sound and ethical clinical trials. The CTN is part of the NAS and was formed in 1990 and subsequently refunded in 1993 under phase II. During phase I, the CTN received $3.4 million per annum; however, this amount has fallen to $2.9 million per annum in phase II. This funding supports the network infrastructure, but does not pay for actual clinical trials. Rather, the existence of an efficient and competent trials network attracts pharmaceutical companies to make use of the network. Dr. Martin Schechter, National Director, CTN, pointed out that the network, in terms of patient enrolment, is one-tenth the size of the United States AIDS Clinical Trial Group, but it operates on 1% of the American Group's budget. Approximately 75% of the CTN budget goes to paying the salaries of its highly trained staff. Clinical trials are sponsored by the international pharmaceutical industry. The provinces, as partners in the CTN, supply some funding, and Canadian granting agencies may fund some specific research projects.
Martin Schechter described the two major benefits provided by the CTN. The first and most important advantage is that the CTN encourages pharmaceutical companies to test their drugs in Canada, thereby bringing some of the best and most promising therapies to Canada as early as possible, so Canadians can benefit. Without the CTN drugs might be tested elsewhere and might remain unavailable to Canadians until after completion of the three phases of clinical trials and regulatory approval by the Drugs Directorate. The second benefit is that the CTN attracts a large amount of pharmaceutical money to Canada. During the last four years, the CTN has conducted 40 clinical trials; and, the five largest trials alone attracted more foreign dollars into Canada than the entire cost of the network to date. It is estimated that the multiplier effect is about a tripling of the federal dollars invested in the CTN.
Although the CTN is doing an excellent job, Martin Schechter and Dr. Michael O'Shaughnessy, Director, B.C. Centre for Excellence in HIV/AIDS, identified two areas of significant concern: an inadequate level of funding and the allocation of funding within the constraint of an artificial five-year plan. The funding level is inadequate from the point of view that while the infrastructure is supported by the federal government, the network must depend upon pharmaceutical manufacturers to fund the trials. The problem is that a pharmaceutical company will only design and conduct trials that are of direct interest and benefit to them. As Martin Schechter explained:
-
Under agenda, there are trials that are important to the quality of life of people with
HIV and that fall outside the interests of the pharmaceutical industry. For example,
I can name the Concord trial, a well-known collaboration between the British and
French governments, which showed use of AZT could be delayed and the same
clinical benefit provided with far less toxicity and discomfort to the patient. That's
the type of trial the manufacturer would not necessarily want to fund, yet it is of
critical importance to people living with the disease.
Many important trials, where we try to find newer, more cost-effective, or less toxic ways of improving the quality of life of people, are not necessarily going to be on the same agenda as that of pharmaceutical sponsors. (3:18)
Also, it is not cost-effective for the pharmaceutical manufacturer to conduct clinical trials in small centres or remote settings, and pressure is placed upon the CTN to restrict trials to the large urban centres. While this action may save money, access to the experimental drug is, in effect, denied to people living with HIV/AIDS in areas distant from Canada's major cities.
Of particular concern to the HIV/AIDS community is the reality that pharmaceutical manufacturers will not conduct clinical trials for agents that cannot be patented. AIDS Treatment News maintains a list of promising agents that need to be studied and are not. There are approximately 30 agents on this list, including vitamins and various micronutrients which are believed to play an important role in the pathogenesis of AIDS. Brian Farlinger of AIDS Action Now! (AAN!) discussed this problem:
-
A very significant problem in Canadian pharmaceutical policy is the continued
absence of a government role in research and development. Pharmaceutical
companies are interested in drugs if they hold the patent rights, the marketing
prospects, the executive commitment, the personnel, capital, facilities and all
other necessary resources to bring a drug to market. The gap in Canada is that
agents which are extremely promising on medical and scientific grounds are
overlooked. (6:46)
In addition, Martin Schechter noted that pharmaceutical companies have indicated no interest in financially assisting the associateship program of the CTN, a program to train outstanding young candidates. Accordingly, the CTN wishes to have its federal funding raised to $5.8 million per annum, the amount identified prior to phase II as that needed for the CTN to operate efficiently and be able to conduct some of its own targeted trials. This sentiment was echoed by Brian Farlinger:
-
In our 1993 document, Confronting the HIV Research Crisis . . . we ask the federal
government to immediately increase the budget of the Network to 5.8 million
dollars per year, so that it can make clinical tests which the pharmaceutical
industry is not ready to do on agents which present scientific merits. The situation
hasn't changed and we reiterate this request. (6:46)
Still on the subject of funding, it was noted that pharmaceutical companies were reluctant to give the CTN block grants so that they might design and operate their own trials. Part of this reluctance was due to the fact that this type of block grant would not be viewed by Revenue Canada as a taxable deduction. Martin Schechter asked the Sub-Committee to look at finding creative ways of giving incentives to sponsors to direct moneys to organizations like the CTN and other disease-oriented clinical trial networks.
The other area of concern is the use of a five-year plan to fund action against a continuing and growing disease for which no cure is in sight. AIDS is not a short-term problem and it is questionable whether it should be tackled by means of a series of short-term funding arrangements. In particular, funding within a specific five-year time frame acts as a disincentive for pharmaceutical companies to use the CTN. For example, a pharmaceutical company is going to have reservations about using the CTN for a three-year phase III clinical trial if Phase two of the NAS is nearing its end and there is no assurance that the CTN will continue in existence. Martin Schechter also pointed out that the field of HIV/AIDS clinical trials and research is less attractive to new, young researchers when they know this discipline has "uncertainty built into it." (3:19) Therefore, the Sub-Committee recommends:
19. That Health Canada reevaluate its funding mechanism for the CTN and
release the network from the constraints of set-term funding. Further, to
alleviate uncertainty, Health Canada should release a statement of
intent in regard to its commitment to continue support of the CTN as
long as there remains a national need to test experimental HIV vaccines
and therapeutic drugs in clinical trials.
Carl Bousquet, President, CPAVIH, requested that federal guidelines be developed and implemented to ensure the participation of women and other underserved populations in clinical trials. Darien Taylor of AAN! requested that the federal government's regulatory agency promote innovative, small clinical trials of promising agents, which could shorten the whole drug review process from years to weeks.
-
With viral-load tests, researchers can now test potential antiviral drugs and
combination treatments in people in small, rapid trials and obtaining results in
weeks. These are known as proof-of-principle trials and they can be conducted
with as few as 10 to 20 participants. (6:48)
In recognition of the value of the work undertaken by the Canadian HIV trials Network and of the need to conduct a broader range of clinical trials which do not necessarily fall within the strict financial interest of the pharmaceutical industry, the Sub-Committee recommends:
20. That Health Canada, in collaboration with all partners involved in the
fight against HIV/AIDS, including pharmaceutical companies, review
the activities of the CTN in order to expand the types of trials;
21. That Health Canada consider the advisability of providing the CTN with operational funding to allow it to conduct autonomous clinical trials on agents that show promise in the fight against HIV/AIDS.
B. Basic Research
The NHRDP has been supporting investigator-initiated research since AIDS was first identified in the early 1980s, prior to the establishment of the NAS. In addition to the efforts of the NHRDP, MRC is committed to providing at least $2.0 million per annum to HIV/AIDS research. Until this year, these two agencies worked independently; however, now AIDS-related grant applications may be submitted twice a year (15 September and 15 March) to the NHRDP and they will be evaluated by a joint NHRDP-MRC review panel. NHRDP-MRC funds go to support research of various forms; however, the largest proportion of grants is directed toward basic, biomedical research, at universities and hospitals. As well, funds are allocated to career and training awards and support for HIV/AIDS research conferences. As of the end of the 1994-95 fiscal year, cumulative NHRDP HIV/AIDS funding has amounted to $49.3 million, while MRC has invested approximately $16.7 million.With the exception of officials from Health Canada and MRC, all of the witnesses who discussed Canadian HIV/AIDS research activities with the Sub-Committee remarked on the inadequate level of funding. Repeatedly, the Sub-Committee was reminded that of the G-7 nations, Canada has the third highest incidence of HIV infection, yet directs the least amount of money to HIV/AIDS research. For example, if the Canadian government adjusted its spending to support AIDS research to the same extent as the U.S., adjusting for our smaller HIV population and weaker economy, Canada should spend about $50 million per year,(86) as compared to joint NHRDP-MRC funding of approximately $7.5 million per annum. AAN! has called for the federal government to make a commitment to long-term, sustained funding of AIDS research proportionate to our HIV population and relative wealth.(87)
(86) Canadian AIDS Society et al., Responding to Emerging Issues in HIV/AIDS Basic and Clinical Science Research, 19 October 1994, 4 p.
(87) AIDS Action Now!, Confronting the HIV Research Crisis: Treatment Activists' Perceptions of the Canadian AIDS Research Effort, Toronto, 12 October 1993, p. 19.
Michael O'Shaughnessy commented on the need for stable, long-term funding. After Phase I of the NAS, HIV/AIDS research funding dropped. "I find it curious that as the epidemic grows, research spending has been concomitantly reduced." (3:22) In addition, current funding levels are only committed until the end of fiscal year 1997-98, after which the future is uncertain.
-
This, I believe, causes uncertainty and inefficiency in the research community
and has an impact on the likelihood that investigators would avoid research
initiatives requiring long-term commitments. Since none of us knows when or
how the scientific solution to HIV will originate, any strategy that reduces these
probabilities is shortsighted. (3:22)
Dr. Mary Ellen Jeans, Director General, Research, Programs Policy and Planning Directorate, and in charge of the NHRDP, told the Sub-Committee that approximately 30% of all the HIV/AIDS research proposals submitted to the NHRDP were recommended for funding by the peer review committees. Further, she was pleased to announce that, until recently, sufficient budget funds had been available to fund all recommended projects. In addition to funding investigator-initiated research projects, the NHRDP also grants career and training awards. The purpose of the career awards is to recognize and reward research excellence, while the training awards are designed to attract and support promising graduate students, thereby promoting and building HIV/AIDS research expertise in Canada. However, according to evidence given by Catherine Hankins (CAHR), in the last round of training grants only about 25% of the recommended candidates were actually funded.
-
I talked with people on both of these committees [NHRDP and MRC] who were
very distraught, because the normal cutoff level in these committees is 80%.
When they're discussing, evaluating, and voting on candidates, their
understanding is that people who get more than 80% during the panel are very
likely to be funded. After the panel was held, the cutoff was changed, due to a lack
of available funds, to 85%. . .
Overall, it means that about one-quarter of those who were recommended by the committees will actually be funded. (2:32)
Michael O'Shaughnessy also expressed his concerns:
-
. . . the long-term implications of this decision may be far-reaching and have
many more adverse repercussions than we might think at present. If the flow of
investigators is restricted, the supply of senior researchers for the next decades
will diminish. (3:21)
. . . the ability of these young investigators to compete for scarce operating dollars is impaired by their lack of experience and track record. Basic scientists fund basic science on the basis of how safe a bet it is to do this and how productive the investigator is. Young scientists have a very difficult time competing for scarce dollars. (3:21)
In essence, an unfortunate "catch 22" situation is being created. Without adequate training awards, fewer Canadian scientists will gain world-class expertise in HIV/AIDS research; and without this demonstrated research excellence, they will not be readily considered for peer-reviewed research grants.
In addition to inadequate research funding and the failure of the NAS to strengthen and build Canada's research capacity, witnesses highlighted the fact that Canadian HIV/AIDS research activities are uncoordinated. Catherine Hankins (CAHR) said that when HIV/AIDS first became a Canadian concern, the federal government showed great leadership in the areas of epidemiology and public health; however, now it is much less proactive. Mary Ellen Jeans (NHRDP) affirmed that in the early years of the epidemic, no one had any idea of what they were dealing with, and it was essential to begin monitoring the spread of the disease and to set up laboratories and start collecting scientific clues. In the intervening years, much has been learned about HIV/AIDS, and she agreed that it is now time to bring all of the players together (researchers, people with HIV/AIDS, treatment activists and government officials) to discuss and establish a coordinated research strategy.
According to Catherine Hankins, the federal government has not been proactive in establishing a research agenda, and by default, HIV/AIDS research has been "completely investigator-driven." (2:29) Investigator-initiated research is curiosity-driven research; that is, the researcher pursues what is of interest or what is thought to be a good idea. Such "pure" research has many benefits and can be very rewarding; however, during periods of emergency, such as the current HIV/AIDS epidemic, its diffuse and potentially scattered response may not meet, in a timely fashion, the specific needs of those afflicted.
The five national partners agreed that there is a pressing need for a national HIV/AIDS research strategy that promotes a good balance between investigator-initiated research and the proactive development of priority research areas. Coordination is needed to build on present strengths and to avoid duplication. Of particular importance is the need to develop collaborations, where teams of research groups target a priority area, for example, gene therapy. Finally, it is important that Canada share research advances internationally, by such means as technology transfer, the training of personnel, and providing advice on research design and implementation.
On the point of establishing a list of research priorities, there appears to be no disagreement among witnesses on the necessity of having people with HIV/AIDS as an integral part of the decision-making process. Carl Bousquet (CPAVIH) observed that, because of their firsthand experience, treatment activists are a valuable resource and should have a recognized role in the setting of research priorities. Both the CPAVIH and AAN! have developed a list of basic and clinical research priorities. In broad terms, the basic-science research priorities include: improved tests for viral load; more study around drug resistance, various opportunistic infections and gene therapy; elucidation of HIV pathogenesis; and more comprehensive understanding of human physiology, particularly of women. Priority clinical research needs include: greater investigation of compounds to boost immune response; target life-threatening opportunistic infections, such as wasting; evaluate gender-specific pharmacokinetics, long-term drug effects and drug interactions.
The need for a national HIV/AIDS research strategy has been discussed by the five national partners with Health Canada, and the government's response has been the development of a document entitled: Toward a National HIV/AIDS Research Planning Process: A Discussion Paper.
-
The concept is to have a forum where partners and stakeholders can discuss
issues at the general level and explore, negotiate and resolve issues that need to
be addressed. The idea is to have a national framework to identify issues that
require attention and determine whether new planning processes are required or
whether there are enough in place already to deal with an issue. (2:31)
According to Catherine Hankins, the five national partners are looking upon this proposal very favourably. They are keen to participate in the process, and to get it going as quickly as possible.
There is considerable disagreement between the pharmaceutical industry and AIDS activists and AIDS researchers on the amount of basic research pharmaceutical companies are conducting in Canada. Judy Erola (PMAC) assured the Sub-Committee that as a result of changes to the Canadian Patent Act in 1987 and 1993, the industry has honoured its commitment to increase R&D spending in Canada. Spending increased from $103 million in 1987 to $504 million in 1993. The Patent Medicine Prices Review Board reported that PMAC (patent-holder) members now direct approximately 11-13% of sales to R&D. AIDS activists contend that pharmaceutical companies operating in Canada focus their research dollars on clinical trials at the expense of basic research. Judy Erola countered this claim, stating that in Canada slightly greater than 25% of all R&D funds are directed to basic research, and this amount is above the worldwide average. Catherine Hankins was able to shed some light on this discrepancy:
-
. . . we know that everything and anything called marketing research counts as
research and development. This does not necessarily consist of looking at things
through a laboratory microscope. They use the expression research and
development to describe the whole marketing side and we hadn't foreseen that.
(2:36)
Darien Taylor (AAN!) called for government action on the issue of what she called "the dearth of basic research in Canada":
-
Concerning basic science, pharmaceutical companies in Canada do very little
basic science research in AIDS. No company conducts in-house basic research,
although some do contract out work to Canadian experts such as the McGill AIDS
Centre. Basic science is usually done in a company's home base and then
related clinical research may be brought to Canada. (6:45)
. . . One would have hoped the extension of patent protection would have resulted in significantly more basic research in Canada. We call upon the government to fulfil the promise. . . of conducting a review of the Patent Act and its pharmaceutical policy. (6:46)
Another area of disagreement between AIDS activists and PMAC is the degree to which pharmaceutical companies consult with the HIV/AIDS community. According to Judy Erola, PMAC has established an Advisory Committee on HIV/AIDS Infection which includes members of the Canadian HIV Clinical Trials Network steering committee. Its role is "to advise member companies on all matters relating to HIV/AIDS therapies, ensuring that member companies are aware of not only the scientific challenges but the social and ethical challenges facing the industry." (6:18) Judy Erola did not indicate how extensively the various pharmaceutical companies use this advisory committee as a resource. However, according to the AAN! report card on the AIDS research efforts of Canadian pharmaceutical companies, only one company, Glaxo, has established a community advisory committee to consult about research plans and to listen and act on community concerns.
Witnesses acknowledged that Canada's expenditures on HIV/AIDS research lag behind that of other G-7 countries, but they also recognized the necessity of spending every dollar wisely. On this point, they all highly commended the recent efforts of Health Canada and the national AIDS partners to come together to discuss and design a new coordinated HIV research strategy. Health Canada and the national partners must resolve the issue of long-term HIV research funding, and come to an understanding of the mechanism and structure by which HIV research will continue to be funded after completion of Phase II of the National AIDS strategy. Therefore, the Sub-Committee recommends:
22. That Health Canada take the leadership in establishing, along with
HIV/AIDS partners and the pharmaceutical industry, a coordinated
HIV/AIDS research planning process;
23. That the prime objectives of this planning process be the effective use of scarce resources through the development of research collaborations, the identification and elimination of duplication, and the development of a list of research priorities for the purpose of targeted funding.
COMPASSIONATE ACCESS
Accessibility to investigational drugs to treat HIV and HIV-related opportunistic infections was a prime concern expressed by representatives of the HIV/AIDS community. Douglas Buckley-Couvrette (CPAVIH) told the Sub-Committee that people terminally-ill with HIV have the catastrophic right(88) to choose whatever therapy they wish, whether that therapy has or has not been approved by the Drugs Directorate of Health Canada.
(88) The concept of catastrophic rights holds that any catastrophically-ill adult has the right to elect, in consultation with his or her physician, any therapy whatsoever that does not cause direct harm to others.
A pharmaceutical manufacturer may not market a drug in Canada until it has received regulatory approval; however, there are three mechanisms by which catastrophically-ill people can obtain access to an experimental drug. First, an individual may volunteer for a clinical trial in which the efficacy of the investigational drug is compared to either a placebo or a standard therapy. In this case, the volunteer has no idea if the drug is or is not the experimental therapy. This should not be a problem if the individual volunteered for the purpose of furthering scientific knowledge. Aids Action Now! objects to this type of clinical trial and believes ". . . that everyone should get some kind of treatment. Certainly people should not be forced to enter a trial as their only hope of getting the drug."(89)
(89) AIDS Action Now!, Aids and HIV Drug Trials in Canada - What You Need to Know, 3rd Edition, Toronto, April 1993, p. 6.
In Canada, experimental drugs may also be obtained by two types of compassionate access. According to Darien Taylor of AAN!:
-
Compassionate access refers to the provision [by a pharmaceutical
manufacturer] of a therapy that has not yet been approved for marketing to
people who are ill and who in consultation with their doctor believe it may offer
hope of saving a life, re-establishing health, or alleviating suffering. This is
obviously a risky therapeutic strategy, but usually these people have failed all
other alternatives and are excluded from participating in the clinical trial of the
same agent. (6:46)
One way of obtaining compassionate access is to join a clinical trial that has an open arm. In this type of trial there are two arms, the double-blind study (controlled arm) and an open arm. People who either did not wish to join or perhaps did not qualify for the controlled trial may choose the open arm and receive the experimental therapy, usually free of charge. This ensures that only true volunteers participate in the double-blind study, thus helping to guarantee test loyalty and the honesty of test subjects.(90) This point of view is countered by the concern that few people will be motivated to enter a controlled trial if they can be assured of receiving the drug in the open arm. It is argued, if too few people are attracted to the controlled arm, the validity of the trial could be compromised, the trial process slowed, and regulatory approval delayed. Obviously, the only way this question will be resolved is by conducting clinical trials with open arms,(91) an activity which is occurring.
(90) AIDS Action Now!, Draft Model for Pharmaceuticals on Compassionate Access and Clinical Trials, 1 November 1994, 5 pages.
(91) M.T. Schechter, Open Arms and Alternative Clinical Trial Designs, Health and Welfare Canada, Ottawa, 1990, 47 pages.
The second way of achieving compassionate access is by means of a physicians request to Health Canada's Emergency Drug Release Program (EDRP). This program was established because physicians occasionally require drugs not approved in Canada to treat patients with a serious or life-threatening illness when conventional therapies have failed or are unsuitable. In such situations, the Drugs Directorate has a mandate to authorize the sale of these drugs to physicians. Generally, these are investigational drugs or drugs approved in other countries which, because of lack of demand, never went through the Canadian approval process. Prior to 1990, it was alleged that the EDRP exercised paternalistic control, did not acknowledge the rights of the catastrophically ill, and at times would not authorize the release of unapproved therapies to people with AIDS.(92) Today, it is claimed that a request for an HIV-related medication is seldom denied. Indeed, Darien Taylor (AAN!) reported that proposed reforms for the EDRP would see ". . . the government devolving its role in providing access to one of monitoring." (6:51) In essence, this would be similar to the system in effect in the United Kingdom where the physician has the right to exercise clinical judgement in the treatment of his/her patient; that is, the physician deals directly with the pharmaceutical manufacturer in making a request for compassionate access to an unapproved drug.
(92) J. Dixon, Catastrophic Rights - Experimental Drugs and AIDS, New Star Books, Vancouver, 1990, p. 48-52.
At present, the role of the federal government in compassionate access is one of authorizing, not commanding, the limited distribution of an unapproved therapy. It is ultimately the prerogative of the pharmaceutical company whether they will grant compassionate access, and if they do, whether they will charge for the therapy. Carl Bousquet told the Sub-Committee:
-
These [pharmaceutical] companies often do not provide compassionate access,
even after the safety and efficacy of a drug have been established. This happens
for a number of reasons: they feel that offering compassionate access will prevent
people from participating in controlled tests. Often they cite the reason that they
do not have enough of a compound to be able to distribute it on a wide scale. Or,
as in the case of Serono, they don't feel they can afford to give away a drug, even
though it has life-saving potential. (6:37)
Judy Erola (PMAC) explained to the Sub-Committee that everyone in the system from research scientist to dispensing pharmacist has to be paid, and that the pharmaceutical manufacturers "make no apology for making a profit." (6:31) Indeed, without the profit motive there would be no international pharmaceutical industry, and currently there would not be 107 AIDS-related therapies, including 11 vaccines, under investigation.
On the question of timely availability of new drugs to catastrophically-ill people,Dr. Michael Levy, Glaxo Canada Inc., expressed the point of view that "[t]o us, the best kind of access is to have the drug approved by Health Protection Branch so that it's truly available." (6:28) On the issue of the Canadian drug approval process, Judy Erola observed that, of the countries in which PMAC members submit drug approval applications, Canada's review system is the slowest. The Sub-Committee later learned that the Canadian drug approval process has undergone reform for the purpose of accelerating the rate at which new drug submissions are evaluated. In addition, a new system has been established that gives priority evaluation status (fast-tracking) to drugs represented for the treatment of serious, life-threatening or severely debilitating diseases or conditions. Dann Michols, Executive Director, Drugs Directorate, stated that the time from drug submission to final approval in Canada is now as good as the world average, and in many instances superior. He also noted that the major reason for protracted evaluation periods was the submission of poorly prepared and inadequate drug applications. Although not discussed, there was the hint that a poor working relationship exists between the Drugs Directorate and the pharmaceutical industry, a situation that would not be in the best interest of those seeking prompt access to new therapies to treat catastrophic illnesses.
AIDS activists from both AAN! and CPAVIH entreated the government to move beyond their present role of simply allowing access to investigational drugs to one of compelling access. These groups provided a number of suggestions on how compassionate access might be encouraged. Carl Bousquet stated:
-
We believe that the Drugs Directorate or another regulatory agency has a
responsibility to ensure that the pharmaceutical industry fulfils its scientific and
moral obligation to provide a therapy through compassionate access after its
safety and efficacy have been demonstrated. There should be a government
policy stating that unless and until a pharmaceutical company releases an agent
through compassionate access, no further controlled trials of the agent will be
allowed. Again, the government already has some influence in this matter
through the research and development tax credits for the pharmaceutical
industry and the upcoming evaluation of Bill C-91 [Patent Act]. (6:37-38)
Before a pharmaceutical company may conduct a clinical trial in Canada, it must submit available drug data and a trial protocol to the Drugs Directorate for approval assessment. Brian Farlinger (AAN!) stated that it is at this point that action can be taken to encourage compassionate access.
-
Essentially we're asking that at the same time the government reviews a protocol,
a phase II or phase III protocol, approves the design of the scientific arm, it also
asks about the company's intentions with respect to compassionate access and
that the regulatory authorities should make a determination as to whether or not
the proposed compassionate access is reasonable and fair in the circumstances.
If a company is unwilling to provide compassionate access, then we are
recommending that the government not approve the design of the phase II or III
study. (6:50)
Darien Taylor noted that the Canadian HIV Trials Network has already adopted a policy requiring a statement of intention on compassionate access for all phase II and III trials that they sponsor. This mechanism will only act as an effective lever when dealing with companies that wish to conduct trials in Canada. It will not compel companies doing trials elsewhere to provide compassionate access to Canadians. Acknowledging his proposal as draconian, Brian Farlinger suggested that there was a mechanism to compel the other companies to provide compassionate access to Canadians. "The ultimate powers you have there are to revoke the patent or take away the marketing rights for another product that the company sells in Canada." (6:51)
AAN! believes that compassionate access might also be achieved through changes to the EDRP:
-
The federal government should add an advocacy power to the mandate of the
Emergency Drug Release Programme and a disciplinary mechanism to give
pharmaceutical companies an incentive to comply with an EDRP request for
release of an experimental therapy.(93)
(93) AIDS Action Now!, Confronting the HIV Research Crisis: Treatment Activists' Perceptions of the Canadian AIDS Research Effort, 12 October 1993, p. 21.
However, since EDRP reforms are tending toward deregulation; that is, the transfer to physicians of its power to authorize unapproved drugs, it would seem inappropriate, in the context of compassionate access, to give the EDRP either advocacy or disciplinary roles.
POVERTY AND DISCRIMINATION
- What is becoming increasingly clear is that the HIV/AIDS epidemic follows the
path of least resistance. Some of the major societal risk factors include belonging
to a population that is living in poverty or that may be discriminated against and
marginalized. (Diane Marleau, 1:11)
There is no doubt that poverty is a risk factor. There is no doubt that being in a relationship where you are powerless is a risk factor. There is no doubt that being a disadvantaged member of our society is a risk factor for this disease. (Dr. Brent Kvern, CFPC, 5:26)
A. Poverty
As for nearly all diseases, there is a causal relationship between socio-economic inequalities and HIV/AIDS. Studies confirm that AIDS patients living in poverty die sooner than their peers who enjoy more stable economic conditions.(94) What is more, living with AIDS often accentuates the problems of poverty:
(94) Robert S. Hogg, Steffanie A. Strathdoe, Kevin J.P. Craib, Michael V. O'Shaughnessy, Julio S.G. Montaner, Martin T. Schechter, "Lower Socio-Economic Status and Shorter Survival Following HIV Infection," The Lancet, 2 October 1994, Vol. 344, p. 1120-1124.
- HIV infection has a profoundly negative economic effect on many individuals and their
families because: the debilitating effects of the disease erode the ability of individuals
to continue to live independently and to provide financially for their own needs and the
needs of their families; the complex, disabling effects of HIV disease create an
additional financial burden. Basic necessities like adequate food and nutrition,
housing and medication are far beyond the financial means of many people living with
HIV/AIDS.(95)
The more the disease progresses in individuals, the harder it is for them to provide for their own needs. They need a nutritionally enhanced diet rich in proteins. They must often obtain nutritional supplements and vitamins. Infected individuals must also consume vast quantities of drugs during their illness to combat opportunistic infections. These nutritional supplements, vitamins and HIV medication are very costly.
(95) Canadian AIDS Society, Brief, 15 February 1995, p. 23.
Brian Farlinger of AAN! testified before the Sub-Committee that he was living with AIDS and had to spend about $24,000 in 1993 on antiviral drugs to prevent opportunistic infections (6:54). Carl Bousquet of CPAVIH talked about Pfizer's Fluconazole, a drug often prescribed in cases of candidiasis, one of the most frequently encountered opportunistic infections in HIV infection. He indicated that the total cost of an average 60-day supply is $951.60. Mr. Bousquet further explained that if the micro-organism responsible for the infection develops any resistance, treatment must continue for six months at higher doses, frequently in combination with one or more other drugs and thus at very high cost (6:36). He also discussed Serono's growth hormone, which is used in cases of HIV-related wasting. As mentioned earlier, this hormone is available through the EDRP. However, the EDRP has no authority to regulate costs, as a result of which price-setting is left entirely to the manufacturer's discretion. It costs a total of $17,220, based on $205 a day, for the minimum treatment of 6 mg per day for 12 weeks (6:34).
Witnesses explained that most persons living with AIDS contract the disease when they are still very young, that they generally hold low-paying jobs and that they have little or no savings. The vast majority do not have a private health insurance plan. It appears that the situation is difficult even for those who do have insurance because they must cope with repayment waiting periods and must pay the deductible portion not covered by drug plans.
Witnesses stated that persons with HIV/AIDS who hold low-paying jobs and are unable to pay for their nutritional supplements and medication are often forced to leave their jobs prematurely and go on social assistance because that is the only way they can qualify for provincial drug insurance programs. However, the Canadian AIDS Society believes that social assistance benefits (even with the disability supplement) are not enough to pay all basic costs such as housing, food and clothing, in addition to the full range of medication. People living with HIV/AIDS sometimes must choose between taking the appropriate drugs and meeting other equally essential needs because they cannot afford both.(96) Furthermore, provincial drug insurance plans do not necessarily cover nutritional supplements and vitamins,(97) and those plans do not reimburse claimants for costs associated with medication obtained under the EDRP (6:36). The Sub-Committee was informed that persons with HIV/AIDS are sometimes hospitalized solely in order to obtain medication they cannot afford. They must sometimes be hospitalized when an infection worsens or cannot be prevented because the drugs are too expensive.
(96) Ibid., p. 24.
(97) Ibid.
Some provinces have reacted to these problems. For example, Brian Farlinger (AAN!) discussed the Trillium program, which has been implemented by the Ontario government and went into effect on 1 April 1995. This is a drug funding program for debilitating diseases under which the patient's contribution is limited to 4.5% of his or her net income. The government will pay expenses exceeding that amount (6:54). The Government of Quebec is currently studying the question of universal access to drugs, but no official position has been taken to date.
Housing costs in large urban centres are another barrier for persons living with HIV/AIDS. In Vancouver, for example, some people living with HIV/AIDS live in hotel "flop-houses." According to the CAS, this is the only accommodation they can afford that allows them reasonable access to the HIV/AIDS care unit at St. Paul's Hospital, the only such facility in Vancouver. The Society indicated that a one-bedroom apartment in Vancouver's west end near the hospital costs about $700 a month. However, the shelter allowance under British Columbia's Disability Benefits Program is $325 a month.(98)
(98) Ibid.
To assist persons living with HIV/AIDS, some community organizations, with contributions from private foundations, provide direct financial aid to buy medication and supplements not covered by government or private drug plans. Others provide food and clothing banks. The organizations have limited resources with which to provide services, where the demand is constantly increasing. For example, the Pacific AIDS Resource Centre in Vancouver has witnessed a 400% increase in its client load since 1990.(99)
(99) Ibid.
The CNA said it regretted the fact that people living with HIV/AIDS cannot use the money they have in their pension funds. According to CNA, the federal government could exercise leadership by encouraging provincial governments to review their pension legislation so as to include exemption clauses to enable these people to withdraw money from their pension funds.(100)
(100) Canadian Nurses Association, Brief, February 1995, p. 10 and 13.
B. Discrimination
During the early years of the epidemic, there were widespread reports of discrimination against people with HIV/AIDS in the areas of housing, employment, health care and education. Fearing that people with HIV/AIDS posed a direct health risk, many individuals called for mandatory testing and various forms of quarantine. As knowledge was gained, it was recognized that HIV is not transmitted by casual contact, but rather by certain high-risk activities. Public health officials promoted education (the avoidance of high-risk behaviours) as the best form of AIDS prevention, while in health care facilities, standard infection control procedures were found sufficient to make occupational HIV transmission a rare occurrence. Accordingly, the philosophy and practice of HIV/AIDS control today is one of taking personal responsibility for one's own health.HIV/AIDS, like any other medical condition, is considered a disability. Disability is one of the grounds of discrimination prohibited under the Canadian Human Rights Act. Anyone who believes that they have been treated unfairly, because they have HIV/AIDS, associate with people with HIV/AIDS or are a family member of someone living with HIV/AIDS, should address their concern to the Canadian Human Rights Commission for investigation.(101) While education and increased recognition of the rights of people with HIV/AIDS may have substantially discouraged overt discrimination, more subtle forms undoubtedly exist. Many of the witnesses from HIV/AIDS community groups indicated that discrimination often goes unchallenged as the individual is simply too ill to seek redress through long and arduous legal processes.
(101) Canadian Human Rights Commission, HIV/AIDS Discrimination - It's Against the Law, Minister of Supply and Services Canada, Ottawa, 1993, pamphlet.
Two areas where HIV/AIDS discrimination remain apparent are in the calls for health care personnel to be tested for HIV, and for immigrants with HIV/AIDS to be denied entry to Canada. The Sub-Committee was repeatedly informed that the chance of HIV transmission from medical professional to patient was infinitesimally small, while the cost of testing every health care worker would be enormous. Catherine Hankins pointed out that in medical facilities, alcohol poses a far greater risk than HIV:
-
We're not doing any major screening that I know of for alcohol abuse in
physicians, which I would say is a much bigger problem than anything to do with
HIV in physicians. (2:39)
Similarly, some Canadians wish to deny potential immigrants with HIV/AIDS access to Canada on the grounds that they pose a significant financial burden to Canada's health care system. The Canadian AIDS Society believes that this is an unwarranted exclusion and that each individual case should be judged on its merits.(102)
(102) Canadian AIDS Society, Brief, 15 February 1995, p. 17.
A hazy area of discrimination surrounds claims that some physicians have refused to treat patients with HIV/AIDS. There are unquestionably physicians who, because of personal preferences or beliefs, have practised discrimination by rejecting homosexuals or injection drug users as patients. On the other hand, there must also be general practitioners who, never having had a patient with HIV/AIDS, were not knowledgeable and referred the patient to a doctor with HIV/AIDS expertise. From the point of view of a person with HIV/AIDS, it is certainly preferable to choose a physician who is both knowledgeable and nonjudgmental.
Douglas Buckley-Couvrette told the Sub-Committee:
-
. . . only 100 doctors accept to treat HIV and AIDS in Quebec. (6:42)
. . . let's face it, most doctors who treat HIV/AIDS are homosexual men. That's the current situation in Quebec. (6:43)
While this statement is probably quite accurate, it should also be appreciated that gay doctors probably tend to have a gay client base and when the AIDS epidemic came along, as the front-line workers, they became the HIV/AIDS experts by default.
As mentioned earlier, one of the significant negative impacts that discrimination has on the fight against AIDS is in the area of fundraising. According to Roger Bullock (CANFAR), "[b]ecause of the stigma still attached to the disease, raising funds is a difficult and time-consuming task." (3:7) Indeed, some people may not donate to the fight against AIDS because they feel that people with AIDS are deserving of their fate.
-
Many people feel that there are "innocent" and "guilty" "victims" of AIDS - that
those who have acquired HIV through sexual behaviour or injection drug use
have done something wrong and deserve their fate.(103)
(103) Ibid., p. 25.
Another form of discrimination the Sub-Committee heard about was how marginalization and discrimination acts to predispose specific groups to greater HIV risk. Catherine Hankins (CAHR) touched upon this issue:
-
So how are we going to try to address the real problem? I think the thing that is
most important to realize is that this virus attacks vulnerable populations. They
are vulnerable either because of poverty, discrimination, or lack of access to
health care services, good nutrition, and so on. If you look around the world, in
every society the people who have been most affected by this are those who are
in those vulnerable groups. (2:37)
In their brief to the Sub-Committee, the CAS described some of the many ways that discrimination and marginalization encourage the spread of HIV. Individuals who are the victims of prejudice often internalize the discrimination, thus lowering their self-esteem and making it less likely that they will be motivated to look after their health or be receptive to prevention messages. People who suffer or fear discrimination are likely to be driven "into the closet," so it will be harder to reach them with prevention information. Marginalized groups are less likely to have access to the range of health and social services that are important for HIV prevention (such as confidential testing and counselling, affordable condoms; and drug treatment, including needle exchange). Marginalized groups may have difficulty organizing as a community, which limits their capacity to develop their own programs and to contribute to policy and planning discussions. Sex education and AIDS education in schools, where it exists, focuses on heterosexuals, making it difficult to reach young gay men and lesbians with safer sex messages. Similarly, marginalized groups are not well represented in the images of HIV/AIDS which appear in the media, including safer sex and other educational messages. Consequently, those most at risk are less likely to respond to the message.(104)
(104) Ibid.
The CAS noted that many AIDS organizations are unable to obtain life or disability insurance for their employees. This makes it difficult for them to attract well-qualified candidates, particularly people living with HIV, to fill vacancies. For HIV/AIDS programs to be effective, the participation of these individuals is essential. The CAS concluded their discussion on discrimination by stating:
-
Governments were slow to respond to the epidemic and their response today is
not adequate. It is impossible to escape the conclusion that this is due to the
perception that AIDS is a disease of the marginalized few. We cannot believe that
governments would have responded so slowly if AIDS primarily affected white,
middle class, heterosexual men.
Discrimination has a serious impact on the HIV/AIDS epidemic. Fighting discrimination needs to become one of the priorities of the National AIDS Strategy.(105)
(105) Ibid.