HESA Committee Report
If you have any questions or comments regarding the accessibility of this publication, please contact us at accessible@parl.gc.ca.
|
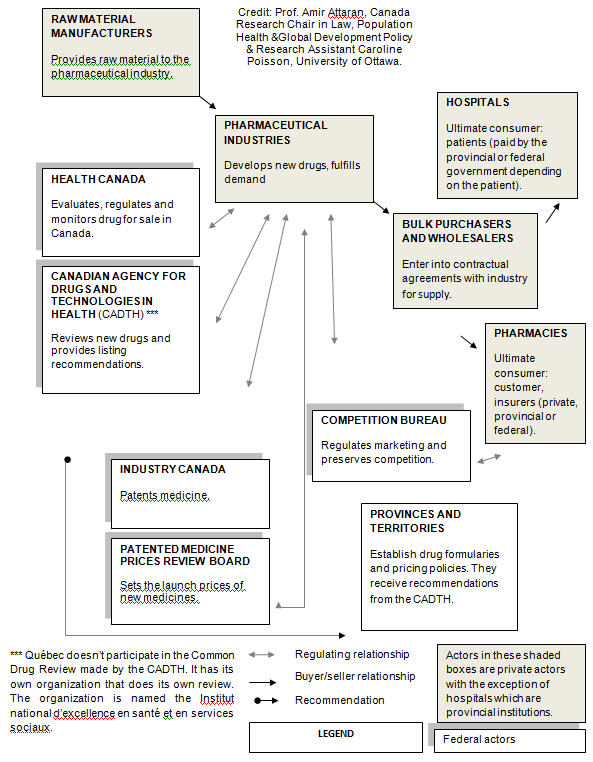
SUPPLEMENTARY OPINION OF THE NEW DEMOCRATIC PARTY OF CANADA Libby Davies, NDP, Vancouver East; Djaouida Sellah, NDP, Saint-Bruno - Saint Hubert; Dany Morin, NDP, Chicoutimi-Le Fjord; and Matthew Kellway, NDP, Beaches-East York. Introduction The New Democrat Members of the Standing Committee on Health are concerned that the final report on ‘Drug Supply in Canada: A Multi-Stakeholder Responsibility’, does not reflect the depth of the ideas shared by witnesses who testified before the Committee. Witnesses told the Committee that the government should focus its efforts on providing solutions to end drug shortages, rather than shifting the burden onto the provinces, territories and pharmaceutical companies. The New Democrat members of the committee understand the importance of federal action on this issue, after putting forward the following motion which passed unanimously in Parliament on March 14, 2012: That, in the opinion of this House, the government should: (a) in cooperation with provinces, territories and industry, develop a nationwide strategy to anticipate, identify, and manage shortages of essential medications; (b) require drug manufacturers to report promptly to Health Canada, the provinces and the territories any planned disruption or discontinuation in production; and (c) expedite the review of regulatory submissions in order to make safe and effective medications available to the Canadian public. The NDP believes that there is a clear federal role in the approval, distribution, and management of drugs in Canada (see Appendix A), as outlined in the National Pharmaceutical Strategy included in the 2004 Health Accords, signed by the provinces, territories, and federal government. As a part of this role, the government must implement the above motion immediately. We are very concerned that government members on the committee looked for reasons to excuse the lack of federal leadership on this issue and minimize the federal role. However, several witnesses identified the federal government as having an important role to play in working with provinces and territories to address drug shortages. We put forward this report to recommend changes the federal government can take to address drug shortages. Dealing with Drug Shortages The Committee was told how the federal government could enact regulations to deal with the current drug shortages. Witnesses outlined proposals for changing the way federal government manages drug shortages, including modifying the current drug acquisition and approval process. They also suggested that regulations used in other countries could improve our current system. New Zealand contractually obligates drug manufacturers to notify its crown corporation, the Pharmaceutical Management Agency, of potential shortages. Manufacturers are also responsible for the costs of sourcing and distributing replacement drugs. In the United States, Congress has introduced the Life Saving Medications Act, which would amend the Federal Food, Drug, and Cosmetic Act to require drug manufacturers to inform of the Secretary of Health and Human Services of potential drug shortages or discontinuations. We urge the Minister of Health and the federal government to work with provincial and territorial counterparts to identify possible alternative sources of critical drugs currently in short supply. We also urge the Minister of Health and the federal government to immediately review Health Canada’s Special Access Program for medications, to suggest changes to the program to approve a high volume of drugs more quickly, to assist in providing critical drugs in shortage. We further urge that Health Canada report to the House of Commons Standing Committee on Health within one year on the progress of the implementation of the motion approved on March 14, 2012, by the House of Commons to establish a nationwide strategy to anticipate, identify, and manage shortages of essential medications. Mandatory Reporting When giving suggestions for how the federal government could better manage current drugs shortages and anticipate future shortages, witnesses repeatedly cited the idea of a mandatory reporting system for drug shortages. Witnesses spoke about how no one was watching out for drug shortages in Canada and that in and of itself, is a fundamental problem. As the Canadian Pharmacists said: what is missing in the drug supply chain is any organization or party that holds accountability for the supply chain from a system wide perspective. Neither government nor any third party has an oversight function for the drug distribution system, and therefore drug supply is dictated in large measure by the market. The Committee heard that a mandatory reporting system could be coordinated by the federal government, and that this system could require drug companies to give notice of shortages on a public website. Witnesses frequently cited that the current voluntary database hosted by third party players was unreliable and did not allow medical professionals access to the vital information they needed. Witnesses also made suggestions about how a mandatory reporting system might work. The system should include a study to identify off-patent drugs that are supplied by one or two companies and that are considered ‘critical’ to medical care and that particular priority should be placed on reporting shortages of these drugs, including greater advanced warning timelines. If companies fail to report supply disruptions, penalties may be applied. New Democrats urge the Minister of Health and the federal government to review the appropriate federal government agency to assume responsibility for a drug shortages notification website, and to work with their provincial and territorial counterparts to set up and provide an investment for a public mandatory reporting system, where drug companies are required by law to report supply disruptions. We also urge the Minister of Health and the federal government to convene an expert committee to identify ‘critical’ drugs and require that any company marketing these critical drugs gives Health Canada a minimum of 6 months warning of supply disruptions. Identifying and Preventing Drug Shortages In the past 3 months we have learned that the federal government could have anticipated and prevented current drug shortages. Records show that in 2008, the Minister of Health was warned about upcoming drug shortages by the Competition Bureau (Benefiting from Generic Drug Competition in Canada: The Way Forward, November 2008). During their presentations to Committee, the Canadian Medical Association and the Canadian Pharmacists Association demonstrated that both of their organizations had conducted surveys in 2011 that showed that doctors and pharmacists were having difficulties dealing with shortages of medications. While we cannot change the unfortunate past actions of the Conservative government, New Democrats believe we can take steps to identify and prevent future drug shortages. Many witnesses spoke about how the federal government could set out mandatory requirements for supply. For example, one of the conditions in granting a Notice of Compliance should be that drug companies submit a risk management plan, identifying the expected demand for their drug and demonstrating its capacity to produce the drug without interruption for at least 3 years. Clauses about notifications of shortages could also be included in compliance and contract requirements and the supplier should also be responsible for locating alternative drugs and covering the costs of substitute drugs should shortages occur. One witness suggested the federal government should study the possibility that it could establish a publicly owned manufacturer to produce critical medications for the country, similar to the government-owned vaccine manufacturer Connaught Labs, allowing it to control the supply of these medications. Witnesses also suggested that the federal government should study the underlying causes of drug shortages, and the impact that access to raw materials, competition, and current drug regulations may have in creating drug shortages. As a representative from Canadian Agency for Drugs and Technologies in Health told the committee: drug shortages are often difficult to predict because manufacturers are reluctant to share details of shortages. This reluctance is largely due to a fear of losing competitive advantage. The NDP also believes that the federal government should begin talks with the provinces and territories to establish a universal prescription drug plan, which would use a common formulary among the provinces and pool the purchasing power of provinces and territories. A pan-Canadian purchasing plan would increase buying power in order to negotiate lower prices for drugs, as well as dividing contracts up amongst several companies to prevent single-sourced suppliers for critical drugs. A pan-Canadian plan would provide Canadians access to a more stable supply of drugs at a more affordable cost. However, the NDP acknowledges, as noted in the 2004 Health Accord, the jurisdiction of the Government of Québec over its healthcare system and that Québec already has its own program in place. New Democrats urge the Minister of Health and the federal government to work with provincial and territorial counterparts to ensure that contracts with drug suppliers include provisions regarding supply conditions. New Democrats further urge the Minister of Health and the federal government to require that drug companies submit a risk management plan when submitting its notice of compliance. We also urge the Minister of Health and the federal government to study the feasibility of a publicly owned generic drug company which would manufacture some of the drugs critical to medical care. New Democrats also urge the Minister of Health and the federal government to convene a study to identify factors causing drug shortages, to determine if there are regulatory measures, in addition to mandatory reporting, that would identify and prevent drug shortages. We also urge the Minister of Health and the federal government work with their provincial and territorial counterparts to create a universal prescription drug plan, to leverage favourable drug supply conditions and improve affordability through bulk buying. Conclusion There is a clear federal role in the approval, distribution, and management of drugs in Canada, which includes enacting the NDP’s motion passed in Parliament to anticipate, identify, and manage shortages of essential medications in this country. There are several suggestions outlined in this report that would help manage and prevent drug shortages in Canada. First, the federal government could create a mandatory reporting site for pharmaceutical companies to report supply disruptions of essential medications. Second, the government could identify alternative sources for essential medications. Additionally, the federal government could take measures to prevent shortages, such as a universal prescription drug plan; studying the possibility of a publically owned generic drug company; or requiring drug manufacturers to guarantee a supply of their drug in drug supply contracts. The final report by government members on the Standing Committee, ‘Drug Supply in Canada: A Multi-Stakeholder Responsibility’ does little to highlight the failures of the current drug supply system. Although the federal, provincial, and territorial governments, along with the pharmaceutical companies, must work together to end drug shortages, it is evident that a lack of leadership has allowed this serious problem to persist. The New Democratic Party, in accordance with the testimony heard from witnesses at the Standing Committee on Health, urges the federal government to take the appropriate steps necessary to identify and prevent drug shortages in Canada. Strong federal leadership can create a new system to resolve the issue of drug shortages. APPENDIX A: DRUG ADMINISTRATION
|